Electronic Patient Reported Outcomes (ePRO)
Capture data at the true source: the patient. Improve the patient experience with secure, accessible questionnaires both via email and through the Castor Connect mobile application.
Schedule demo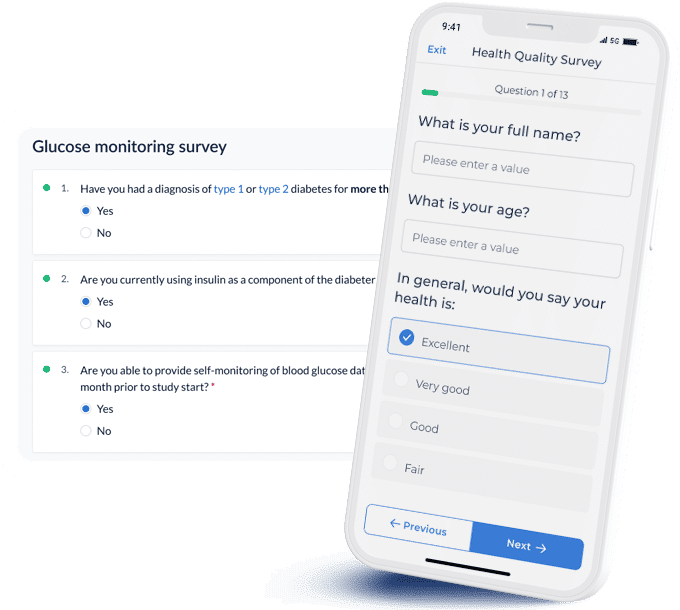
Deploy questionnaires, faster
Create user-friendly questionnaires in minutes.
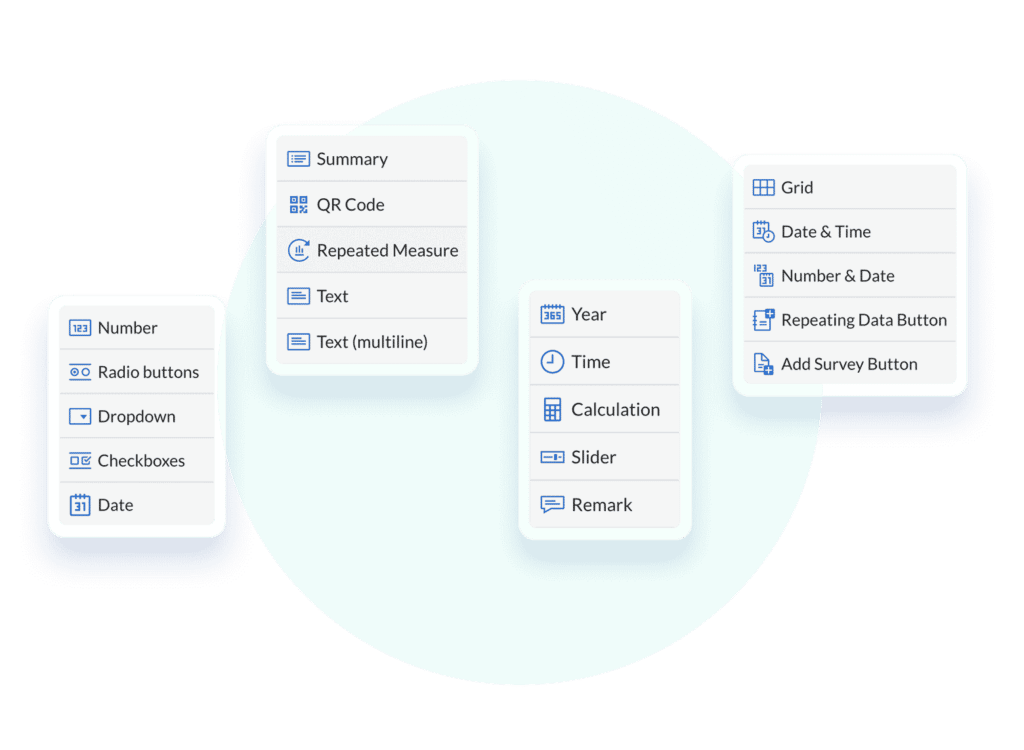
- Select from 21 field types to build your own survey, or get started with one of our validated pre-built templates
- Flexible logic and field validations
- Reduce time spent on rebuilding questionnaires from scratch by reusing existing ones
Increase engagement, remotely
Securely capture quality research data directly from participant mobile devices with the Castor Connect app and track survey progress in real-time.
- Segment participants by study phase or demographics and send bulk survey invites
- Automatically schedule questionnaires via time and action-based triggers
- Track survey results and participant progress on dynamic reporting dashboards
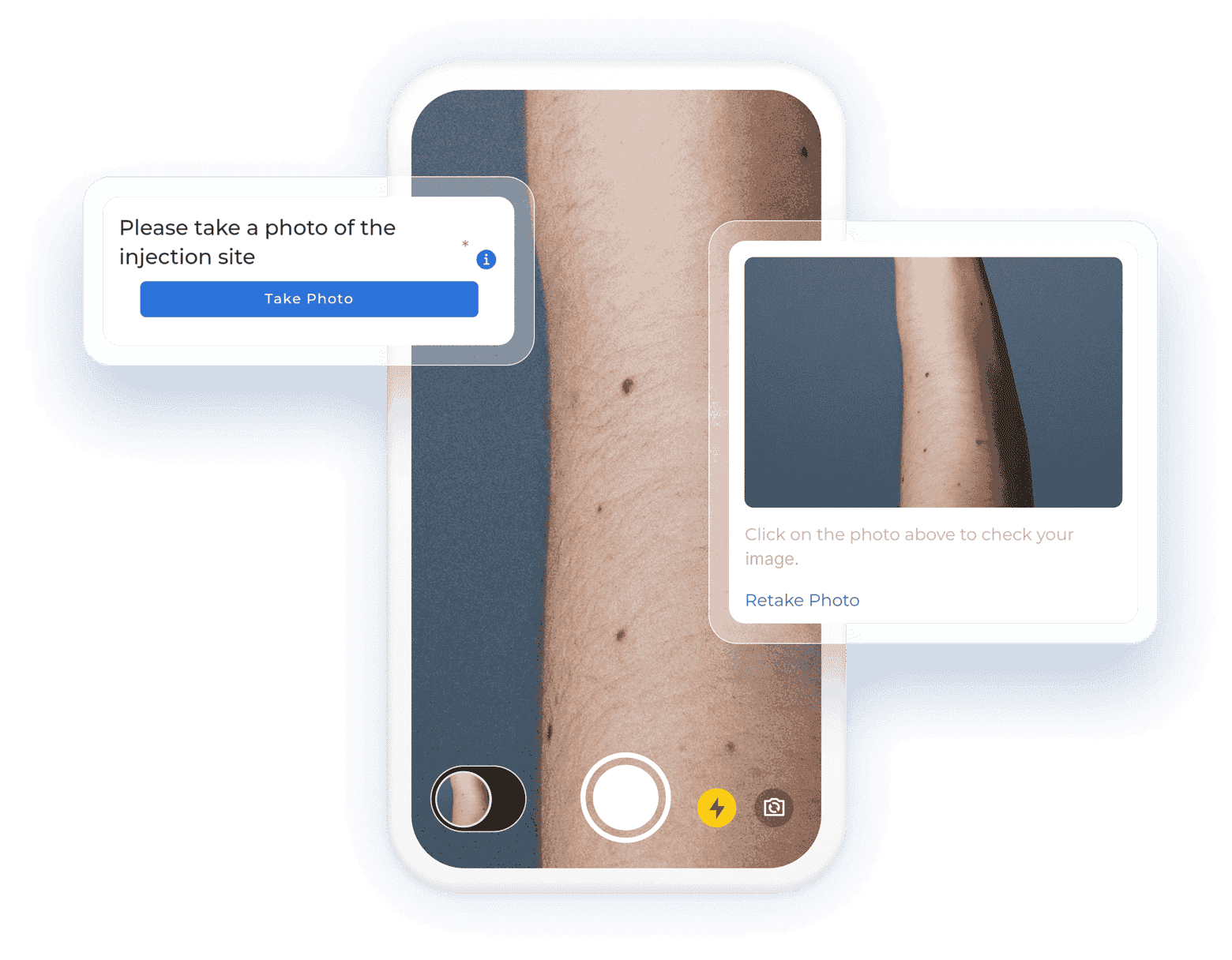
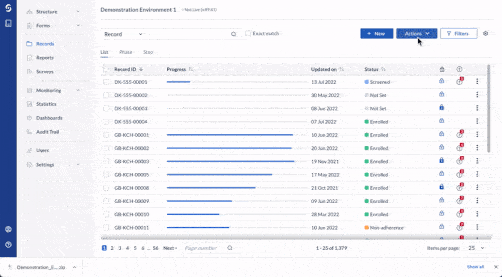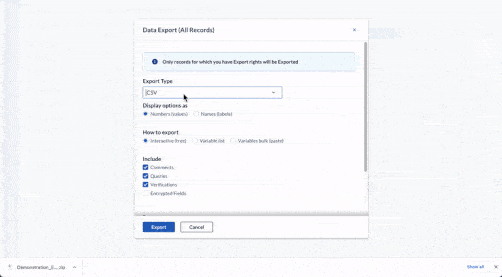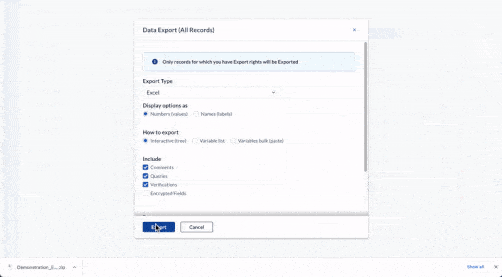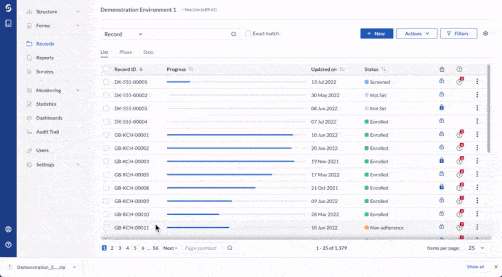
Centralize all data collection
Get a complete, record-level overview of your data in one centralized hub. Be it 10 or 100 questionnaires, unifying clinician, patient, and device data has never been easier.
- Integrate data from EHR, eCRF, ePRO/eCOA, laboratory, wearables, and other mobile devices
- Work with any software or database in your clinical trial ecosystem with Castor’s open API
- Store data securely with 15-year data retention, field-level encryption, and two-factor authentication
Ready to get started?
Generate compliant data, confidently
Our company’s technology and services meet global regulatory and compliance certifications on private and secure infrastructure. Our compliance ensures you have access to the validation documentation needed for each phase of your internal regulatory compliance process.
Bring your own device (BYOD)
Compatible with mobile devices and tablets, build questionnaires in Castor that can automatically be made available in the native iOS and Android app.
All of your data in one place
Switch between email and app capability through a simple checkbox. Castor ePRO and Connect surveys are fully integrated in Castor’s EDC platform and they can be built in EDC or imported from the Castor Form Exchange portal.
Store data securely
Protect your data with 15-year data retention, field-level encryption, and two-factor authentication and store it on certified, compliant servers in any country.
Achieve global compliance
Meet compliance certifications worldwide such as FDA CFR Part 11, GDPR (EU), ICH GCP (HIPAA, US), ISO 27001, and ISO 9001. Align with GCP, HL7 FIHR, and other regulatory guidelines.
Featured resource
AKRN handles mid-study changes in medical device clinical trials
AKRN Scientific Consulting, a medical device CRO, used Castor’s flexible solution to handle constant mid-study changes for their client.
Streamline patient assessments with natively integrated ePRO and EDC
Castor’s ePRO solution enabled AKRN Scientific Consulting to engage with a participant population that, on their own, might struggle with a technology solution that they’ve never used before.
Easy-to-use ePRO surveys, accessible to participants on familiar devices, made real-world data collection possible. Native integration with Castor enabled AKRN to collect and manage data seamlessly, and conserve budget and IT resources that would typically be spent sourcing and integrating with additional vendors.


Native ePRO questionnaire created within Castor EDC

Send email (QR code to download app) or login to mobile site
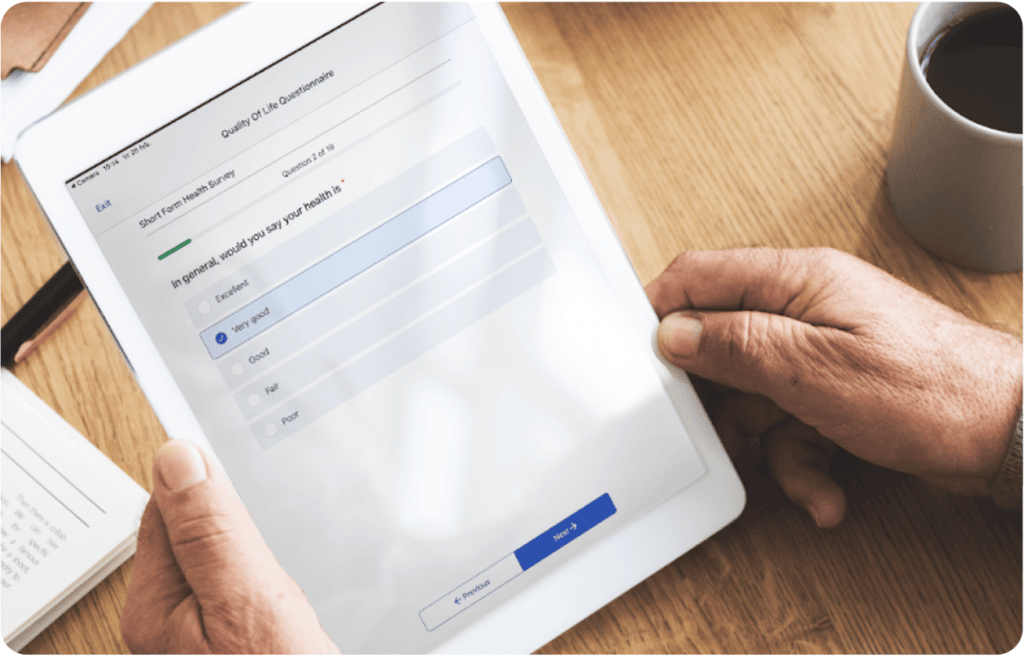
Participants fill out survey on laptop or mobile device
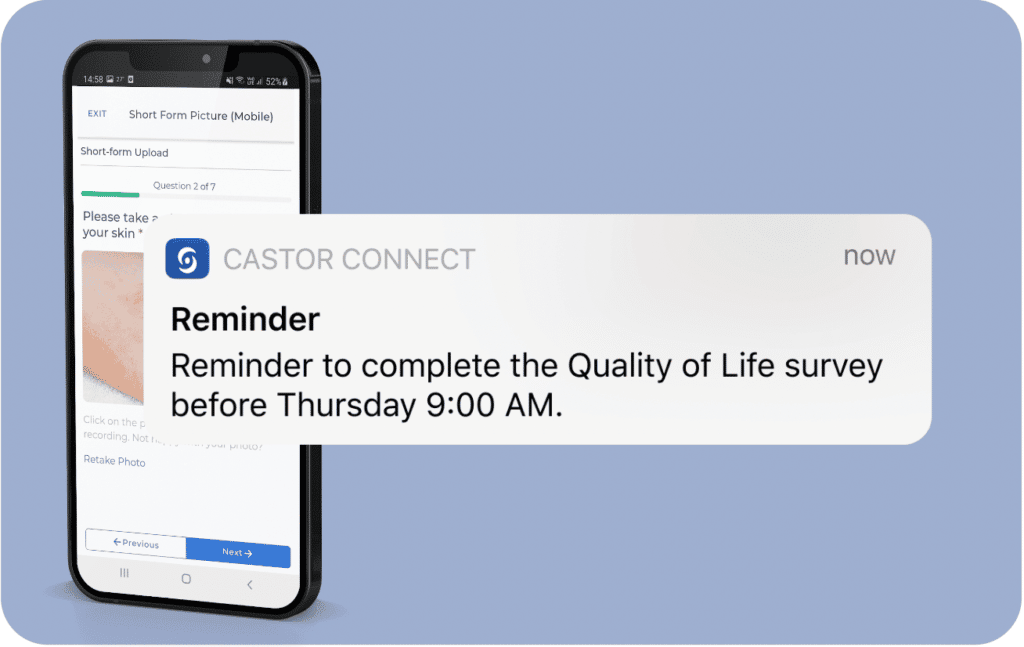
Get app/email notification reminding when to take survey
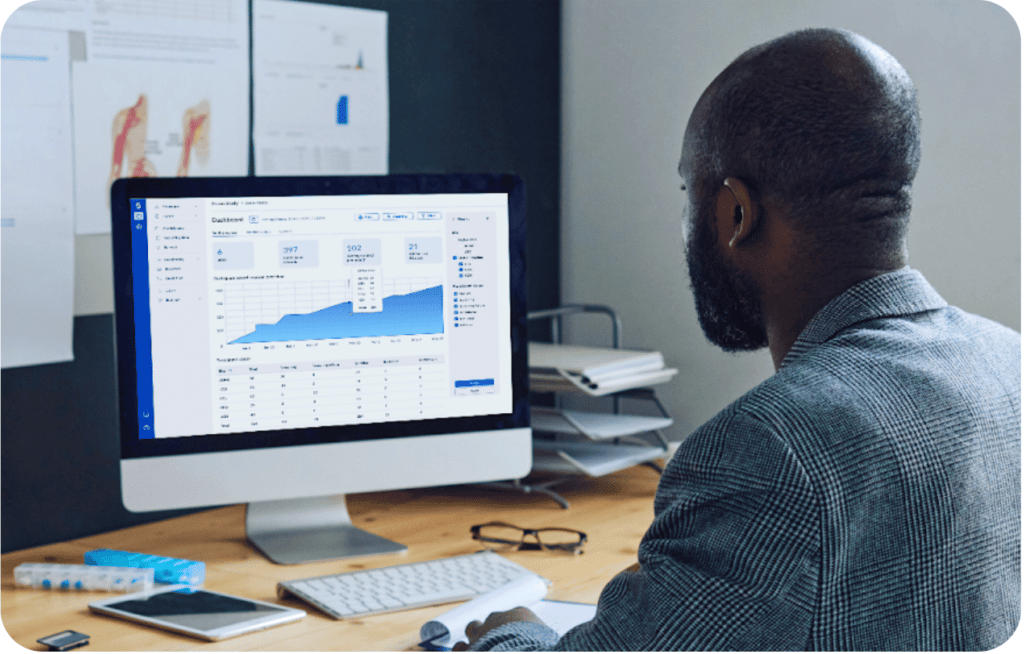
ePRO data captured in Castor EDC
#0
Ranked EDC
0 K +
Happy Users
0 K +
Studies
0 %
Customer Satisfaction
0 +
Countries
0 M +
Patients
0 %
On time delivery
Learn more about collecting patient data with Castor’s ePRO
Over 500 trusted partners







