Maximize productivity and flexibility by capturing, processing, and integrating data from multiple sources on a compliant electronic data capture (EDC) system.
EDC, eCOA & eConsent Solutions for CROs
Castor’s clinical trial platform combines flexibility, interoperability, and a user-friendly interface, enabling CROs to easily scale operations, meet aggressive timelines, and pave the way for growth.
Talk to an expert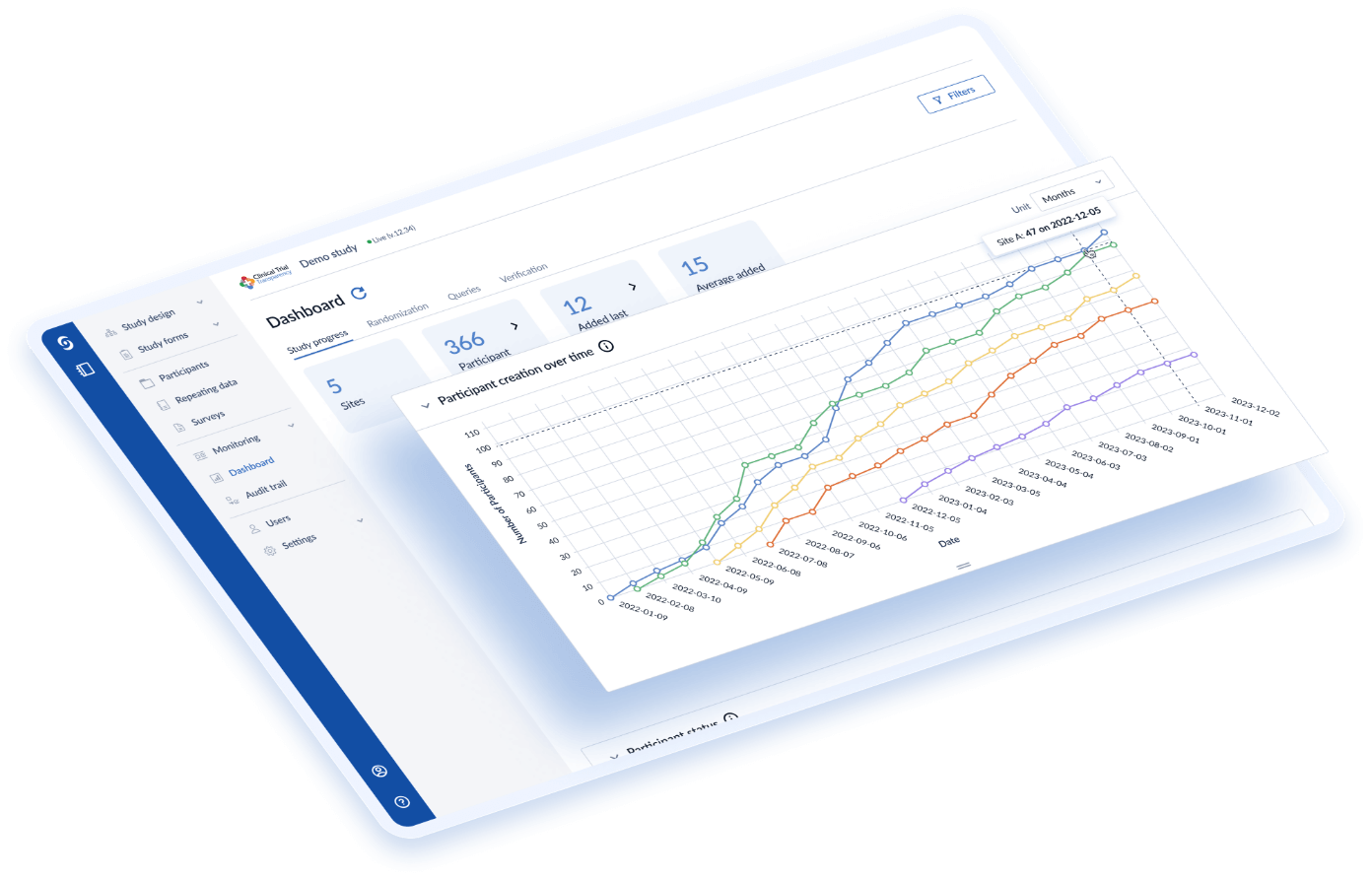
Streamline and scale your clinical trial
Castor’s technology offers Contract Research Organizations (CROs) a practical way to streamline and scale their clinical trials. The modular platform adapts to the unique requirements of each study, enhancing flexibility in your trial design and execution. Designed for ease of use and built for scalability, Castor’s patient-centric solution powers your trials faster, without compromising quality.
Learn how our 147K+ happy users are leveraging Castor
Powerful solutions for CROs
-
ePRO
Say goodbye to paper surveys. Capture and manage patient data right from the source – prioritizing data quality on a centralized platform.
-
eConsent
Create effortless experiences for patients with an all-in-one solution to remotely recruit, screen and enroll patients from the comfort of their home.
-
DCT
Make your sponsors happy with proven ROI. Learn how Castor’s all-in-one DCT platform can help drive efficiencies and patient experience in your clinical trials.
Easy to use, easy to deploy
With over 90% of studies being deployed within 4 weeks, CROs can reduce time spent building and managing studies with the control to quickly adapt to mid-study changes.
Flexibility to scale
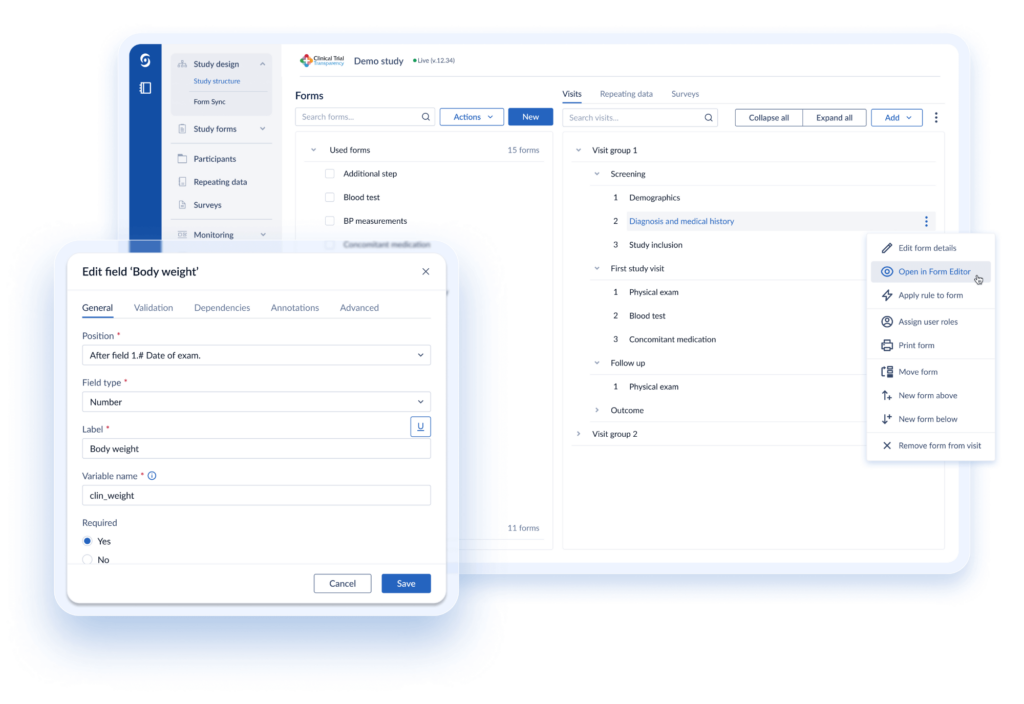
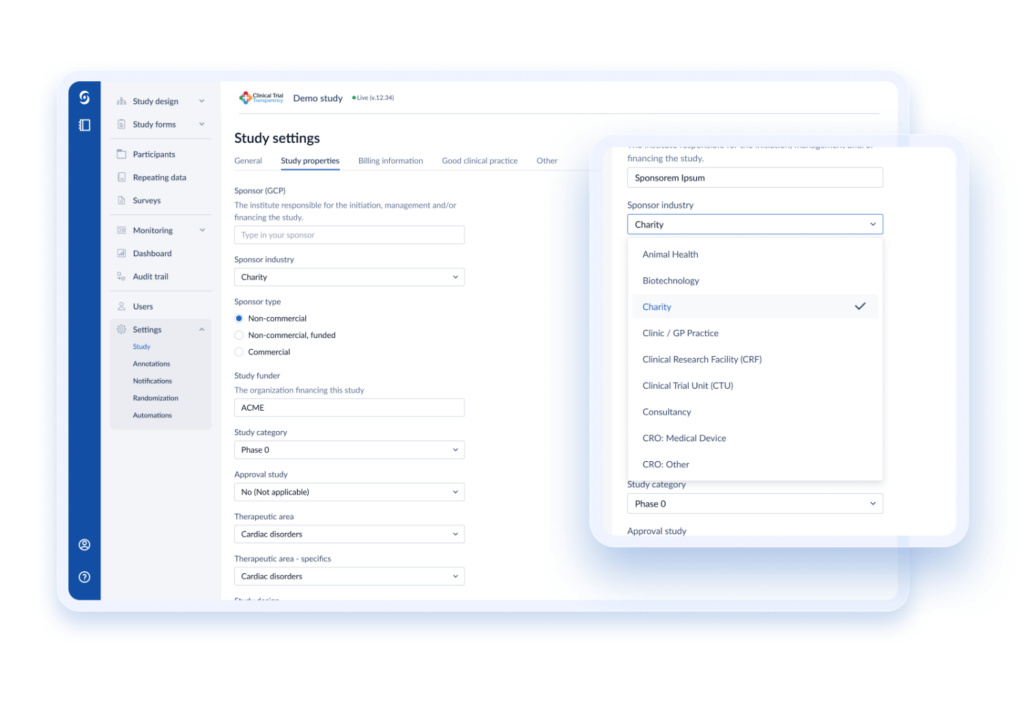
Castor's modular platform offers a flexible approach to study build & design - select only the modules you require. Choose your support level: self-service for complete control, assisted-build for guided support, or a comprehensive Castor-led study build.
Tech first, research always
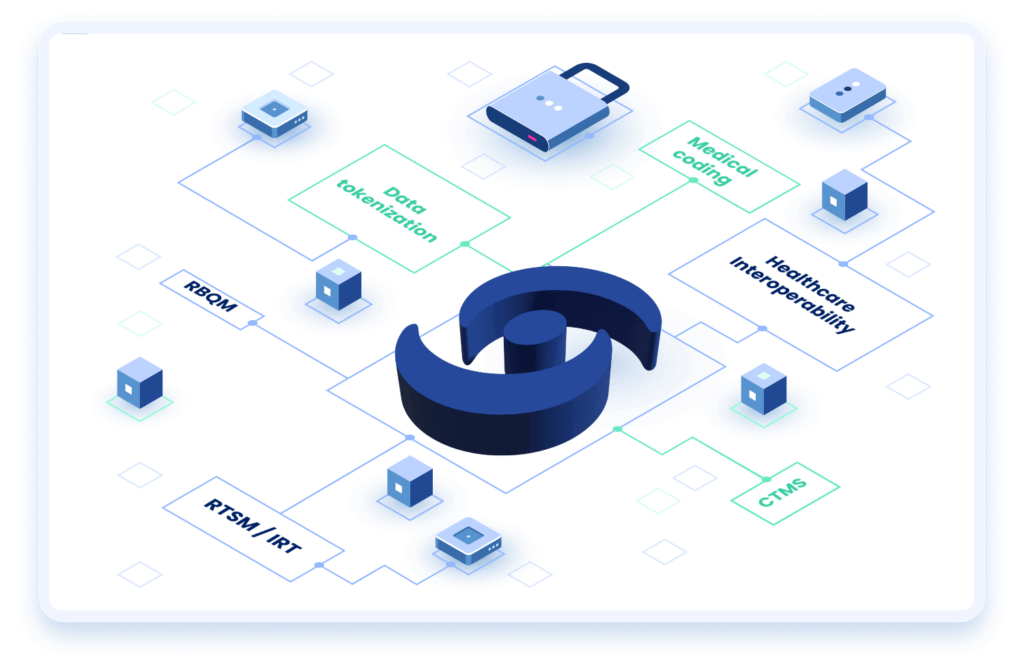
An open API gives CROs the freedom and flexibility to integrate with other platforms, whether in-house, from different vendors, or various data sources, maximizing chances of study success.
Castor gets CROs real results
Castor partners with you from the get-go to ensure that our solutions are optimized to your study design. Having supported over 14K+ studies with over 6m+ enrolled patients, Castor is there for every step, from design to study closure.
Want to know more? Check out our webinars
FDA’s Vision on PRO Collection for RWE: Timing & Methods Explained
Our panel of experts unlock insights on how the FDA view the collection of patient-reported outcomes (PROs) for real-world evidence.
Do CROs Need to Reinvent Themselves in 2025?
Economic constraints, regulatory shifts, and technological advancements are forcing CROs to rethink their value proposition. How can CROs keep up?
$100K+ saved in potential staff costs
“Castor’s all-in-one platform was a game-changer. One coordinator runs our entire study, saving us an estimated $100K in staffing while maintaining real-time data oversight. We’ve had near-complete questionnaire data, and the professional services team has been there every step of the way…”
Dr. Michael Liss
Oncobiomix

Join our partnership program
As a CRO, your needs are unique. That’s why we've built a dedicated program to support your onboarding, product knowledge and much more.

