Castor is heading to San Diego for DIA’s RWE Conference — and we’re not showing up empty-handed. We’re launching Castor Catalyst, our AI agent for real-world data extraction. It’s fast. It’s accurate. And it just might make chart review a little less painful.
📍 Location: The Westin San Diego Bayview
📅 Dates: October 16–17, 2025
🎤 Featured Presentation: October 16 @ 3:30 PM
Castor Attendees:
- Lisa Charlton, VP of Product
- Ashton Choi, Director of Business Development
- Angela Martinez, Solutions Manager


Launching Castor Catalyst: Self-Driving Trials Start Here
On the Road to Self-Driving Trials: Faster, Cleaner RWE Data Extraction
Speaker: Lisa Charlton, VP of Product, Castor
Date: Thursday, October 16
Time: 3:30–4:00 PM PDT
The problem: Chart review is still slow, manual, and error-prone.
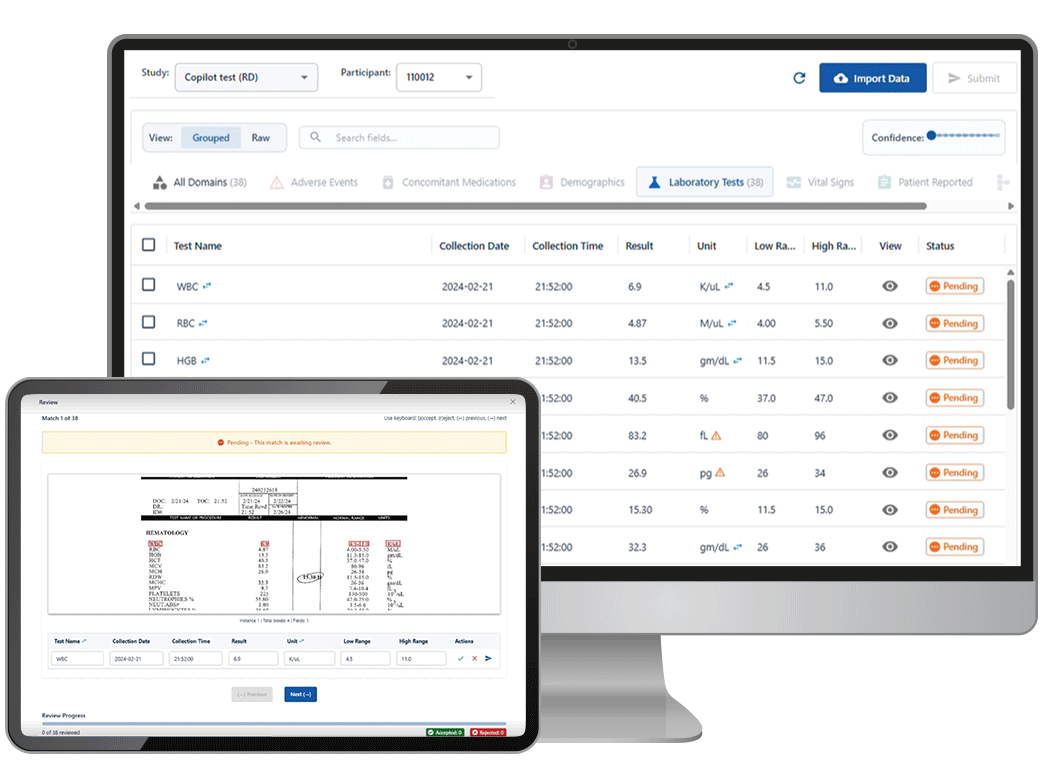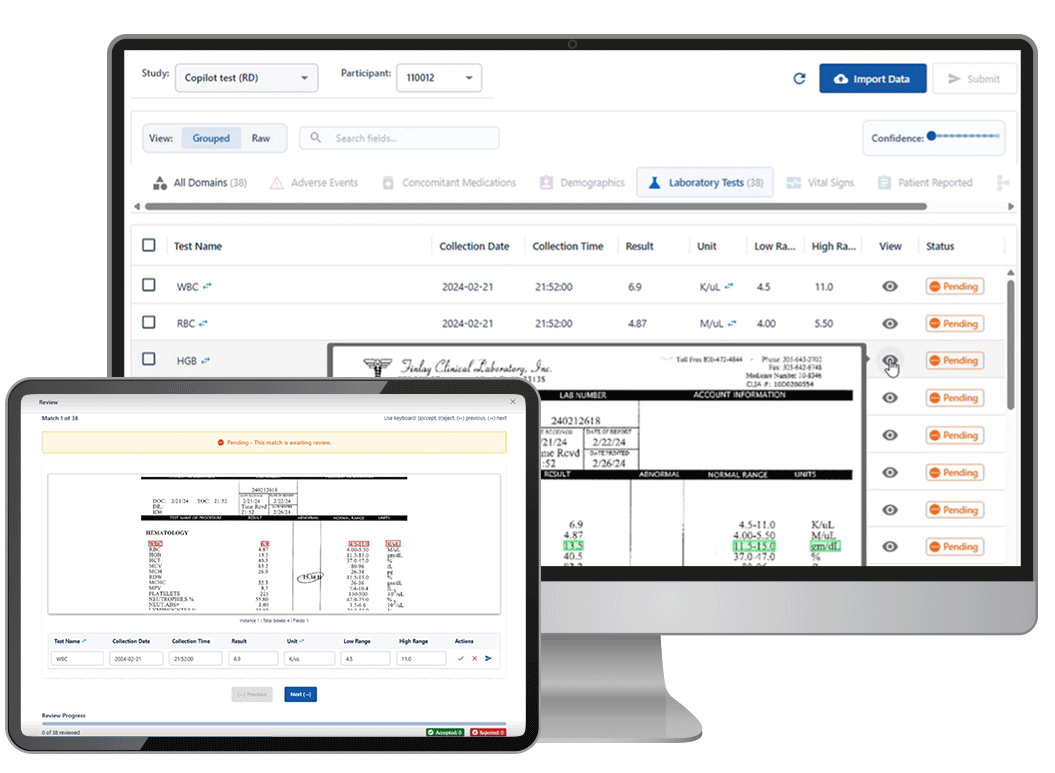
The solution: Castor Catalyst — an AI agent trained to extract regulatory-grade variables from unstructured records and flag exceptions for human sign-off.
What Is Castor Catalyst?
Catalyst is Castor’s new AI-powered assistant designed for real-world evidence teams buried in charts, PDFs, and data chaos. Built for speed. Tuned for compliance.
✅ Extracts structured, auditable variables from unstructured source documents
✅ Flags every change for investigator sign-off
✅ Compatible with existing workflows and oversight models
✅ No endpoint data, no direct patient contact, no autonomy without safeguards
It’s not a black box. It’s a better starting point.
Don’t Miss the Presentation
Be there when we introduce this Castor Catalyst case study to the public.
📍 October 16 | 3:30–4:00 PM | DIA RWE Conference | San Diego
Let’s talk about how to stop drowning in documents — and start getting better evidence, faster.
Why Meet with Castor?
This isn’t just about AI. It’s about doing RWE better — with less friction, faster timelines, and higher quality.
✅ eConsent: Consent patients once, securely, and compliantly — across studies and contexts
✅ ePRO: Real-world data from the patient’s device of choice
✅ EDC: Built to handle hybrid, pragmatic, and registry-based designs
✅ Automation: Get your builds done faster — even with complex protocols
✅ Castor Catalyst: RWE chart review with AI speed and regulatory oversight