Remote desktop and mobile ePRO has been in Castor for years, but in more recent customer conversations, one ask kept coming up: can we extend our current assessment solution to a controlled, on‑site setting?
And that’s what we built. We extended that remote functionality into our core platform for clinicians to access directly. So they can capture participant data in-person while staying aligned with the flexibility and compliance of remote ePRO.
Our recent Product Spotlight with our product experts Christian (Product Manager) and Dualtagh (Manager Solutions Consulting) detailed exactly how the solution works. But in case you missed it or would like a recap, below is an overview.
Flexible data capture for sites and patients
We designed our on-site ePRO to reduce burden on sites without the reliance on hardware. The functionality is modular to our existing ePRO solution. It doesn’t require an extra app or device, and entries flow into your CDMS alongside your other ePRO data.
After starting the on-site session, staff can hand over their device or display a QR code for the participant to continue on theirs. If time in the clinic runs short, progress is saved and the participant can finish up remotely—no duplicate records, no re‑entry. You can switch modes at any time and preserve progress.
“The whole point is that there are different completion options with our on-site ePRO. We know that sites often have a pile of devices at study sites,” Christian explains. “Crucially, our solution is device agnostic. It ultimately scales and flexes to the device that you’re using and that you already have rather than adding yet another thing to that pile of devices at your site.”
For studies
Our On-site ePRO allows for direct and enhanced data capture on the site at FPI. Collecting those ePROs on site at baseline ultimately results in fewer gaps before intervention.
“Missing, inconsistent, or poor quality ePRO data—particularly at baseline—ultimately jeopardizes trial endpoints,” Christian recognizes. “And we all want to avoid that ‘missed data on the patient clipboard in the lobby syndrome’ where they get given the clipboard, enter only parts of the data, they then leave, and have to then come back or be brought back at another point to enter that data.”
For patients
If a participant prefers their own device—even in a controlled site environment—we let them use their own device. Participants choose what’s comfortable in the moment—clinic device or their own—while keeping the option to finish later without starting over.
“It’s much more flexible, it’s kind of part of that broader level of support for decentralized and hybrid trials—complementing the remote data capture and creating that seamless data continuum,” Christian says. “So no matter where they are, no matter what devices they have, no matter what point in the trial they’re at, we can still capture that data safely, securely, and consistently.”
For data managers
On-site ePRO ultimately ensures standardized capture under controlled conditions, improving reliability for regulatory review. Which in turn, improves the overall compliance tracking. Sites can immediately verify data entry, reduce lag, reduce dropout, and have the data sit alongside all of the other data in the same, consistent, compliance report.
Christian concludes, “I think one of the biggest benefits of the module is how wonderfully simple it is. It kinda just works, and leverages our existing assessment technology.”
How it works
As a site user, you can access the solution via a button in the existing platform, opening up a link in the browser that you can bookmark, or add as an icon on your tablet.
After opening the module, you’ll log in and be presented with the on-site administration page, where you select the participant, visit, questionnaire and the participant’s language. Next you’ll verify the participant’s identity and select how to administer the survey.
You’ll be presented with two options:
- Using the site device, where you’ll hand it over to the participant.
- Presenting a QR code on the site device, that the participant can scan to continue on theirs.

Using the site device
Using the site device as a participant, you’ll be presented with a clean and simple interface. You can start navigating through the questions and a progress bar on the left will provide you with an indication of the completion percentage.
“While I’m completing the survey as a participant, that data is auto saving immediately,” Dualtagh explains. “It’s syncing back with the participant’s record within our overarching CDMS. So it’s making sure that data is immediately available for the site to review as well. At the site, and against the record.”

When the participant is finished, they confirm that they’ve completed their responses and will be presented instructions around returning the device. When they confirm and hand the device back, the site user will be logged out to prevent the participant from seeing any information.
The site user can then log back in and will be taken to the administration page again, where they can select the next participant and move forward.
Using the participant’s device
When using the participant’s own device, they’ll scan the QR code presented on the site device.
Just like with the site device, the participant is presented with a clean and simple interface they can navigate through, and the data is auto saving and syncing back to their record.

“It’s just another way in which we can provide that little bit of extra flexibility, for data capturing scenarios where you don’t have that site-based device available,” Dualtagh highlights.
Using remote back up
When you make use of any of our remote back-up options, the participant will be emailed a link to the questionnaire where they can pick up where they left off.
Tracking compliance
Within our CDMS, the compliance dashboard gives you a quick overview of the overall compliance across participants in the study. You can use filters to drill down into the compliance in the last 7 days, 30 days, or all time; or, for example, hiding the 100% compliance entries.

You can use more detailed filters to drill down into data, for example based on specific site statuses, or a compliance percentage window.
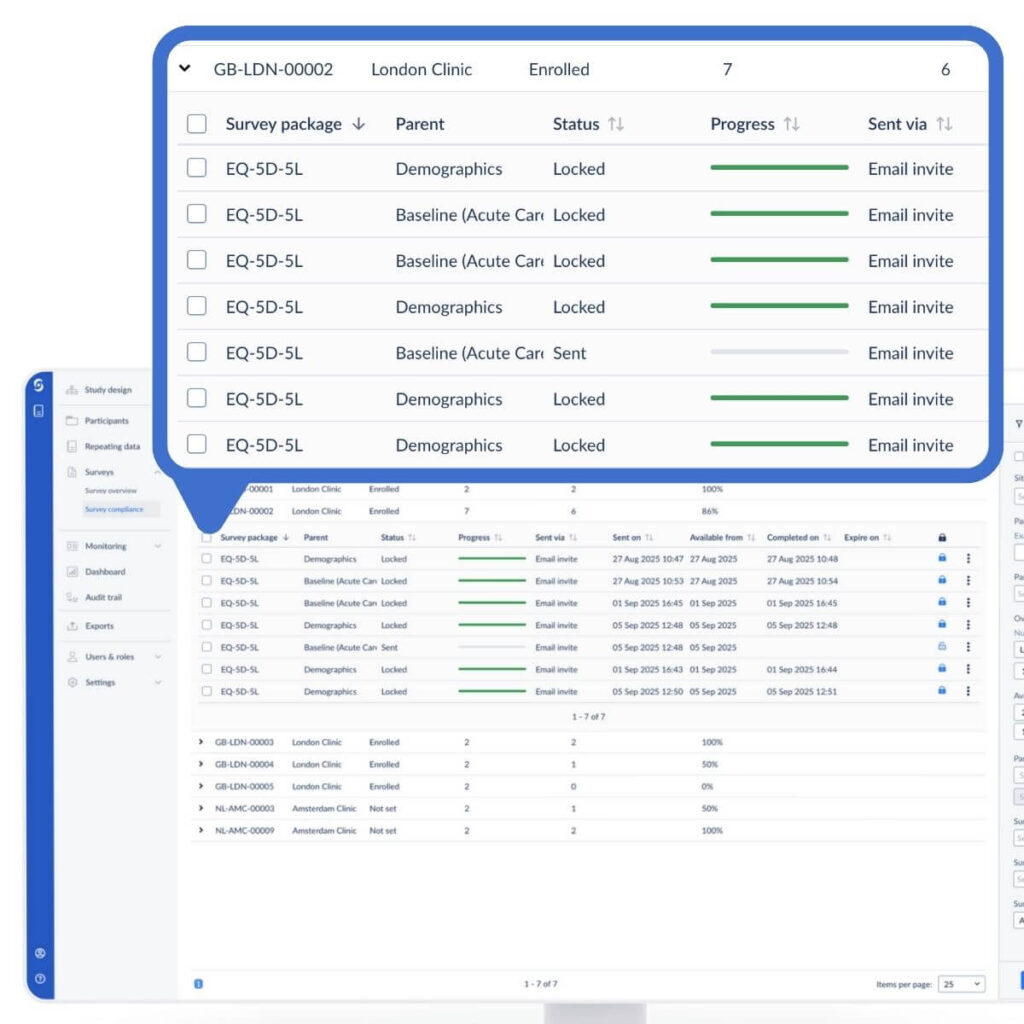
You can also have a look at the specific surveys that have been sent, and dig into the participants to follow up with to ensure good compliance across your study.

“And this really just goes alongside some of our broader functionality for patient reported outcomes,” Dualtagh says. “It sits nicely alongside things like our patient reminders and different notifications for different modalities. So, the ability to remind patients via SMS, via web, via WhatsApp, and these different means to keep them engaged.”
Find out more about Castor’s On-site ePRO
Want to know more? We’re happy to answer questions or get you set up.
For existing customers, studies, and researchers:
The On-site ePRO module can be activated by your account manager. Contact them directly or email [email protected].
For new customers, studies, and researchers:
Contact the Castor team here to get started with Castor ePRO, or email [email protected].
Of course, you can also watch the webinar here.