 This is the second interview in a series of Castor user interviews. This time we speak with Petra Jongmans, Clinical Research Manager at Atos Medical. Petra used Castor to conduct a study with one of their medical devices used by people with a Total Laryngectomy.
This is the second interview in a series of Castor user interviews. This time we speak with Petra Jongmans, Clinical Research Manager at Atos Medical. Petra used Castor to conduct a study with one of their medical devices used by people with a Total Laryngectomy.
“Castor’s competitors did not have such an agile mindset.”
Atos Medical is the world leader in Laryngectomy Care
Atos Medical first introduced their line of voice prostheses 25 years ago and has since become the world leader in Laryngectomy Care. It has developed their devices working together with renowned institutes such as the Netherlands Cancer Institute and is continuously improving their devices and testing them through clinical trials. To be able to deliver world-class devices, Atos Medical attaches the greatest importance to professional clinical trial processes, regulatory compliance and high quality research data.
“Using the tool itself to construct the forms was much easier than anticipated.”
Previously, Atos Medical had conducted their trials using paper because most Electronic Data Capture (EDC) applications were perceived as too cumbersome to set up, both technically and in terms of contracting. Castor delivered quickly and proved very affordable, while complying with all regulatory requirements (e.g. Good Clinical Practice).
“Competitors of Castor are used to working with Pharma organisations and do not have an agile mindset, they offer less freedom to set up the study yourself, and charged a much higher price.”
Quick study set-up and increased efficiency
Compared to creating an annotated paper Case Report Form (CRF) that feeds into an SPSS database, Castor proved to be much quicker to set up. “Our data manager mentioned that using the tool itself to construct the forms was much easier than anticipated. Also, whenever she had questions she received an answer within 2 hours from the helpdesk.”

Easier for researchers at no cost to trial participants
Instead of investigators having to search for a paper folder, they could simply bring their laptop to an interview with a trial participant and get started right away. “Since data entry is so straightforward it was easy to maintain the personal connection with the participant,” says Petra.
Due to the advanced field validations the quality of collected data was boosted because no values outside set ranges could be entered. “After data entry, data can easily be exported to SPSS for analysis. Data Managers save about half a day per patient because they no longer need to manually copy data from the paper CRF.”
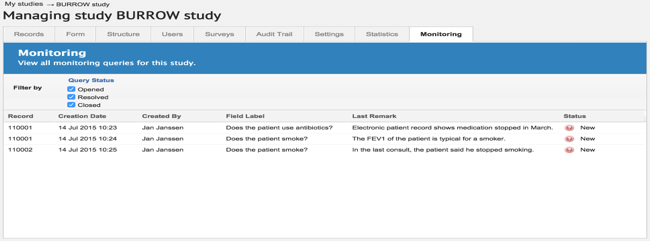
The monitoring work associated with the trial was sharply reduced
Due to the higher data quality, the staff monitoring the trial had fewer queries. “When queries did come up, they could easily be connected to the relevant field and answered promptly using the query overview.”
Results of the trial are still pending analysis, but Castor will definitely be used again!
At the time of writing the trial is still running and in a few months time the results will be analysed. “However, we can already say for sure that we will be using Castor again for any new trials that we conduct!”
Also ready to switch from paper and get your efficiency gains?


