Industry veterans including Craig Lipset will support Castor’s vision to advance the future of clinical research
Amsterdam, Netherlands and Hoboken, New Jersey: November 18, 2019: Castor, a health-tech company that aims to leverage machine readable data to increase clinical trial efficiency, today announces the formation of an independent Advisory Board.
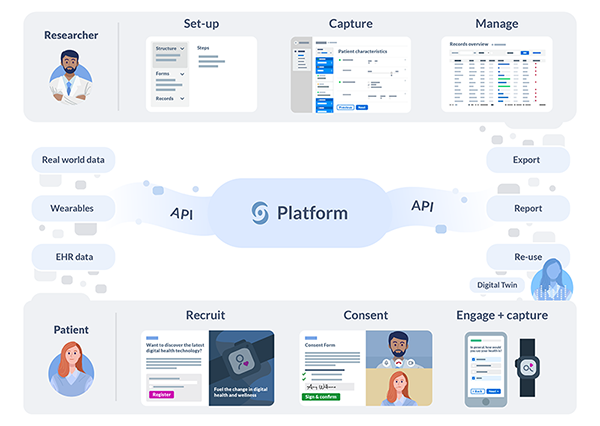
Castor’s Electronic Data Capture (EDC) platform enables every researcher worldwide to easily capture and integrate medical research data from any source in real-time, including clinicians, patients, devices, wearables, and electronic health record (EHR) systems. Researchers on the platform generate vast amounts of data from traditional and decentralized trials, and Castor recently reached milestones of 100,000,000 data points and 1,000,000 enrolled patients.
Over the past few months, Castor’s management team consulted with several experts in the sector to establish the Advisory Board, bringing together top executives and key opinion leaders in the field. The new members will provide invaluable expertise and guidance to help the company achieve its vision of making the world’s research data reusable, enabling AI-driven clinical trials, and ultimately creating a future in which we maximize the impact of data through reuse.
On welcoming the members, Castor CEO Derk Arts, MD, PhD, said: “We are delighted to have attracted this prestigious and diverse group of experts from international institutions to our Advisory Board. This independent body will help us accelerate the development of our platforms, forge new commercial partnerships worldwide, and realize our vision of helping advance medical research. The Board members will bring significant expertise, highly complementary skills and an external perspective, which will be invaluable in informing our strategy as we move forward.”
Craig Lipset, newly appointed Castor Advisory Board member, commented: “Castor has built a platform around a unique vision to transform how medical research data is captured and shared. I am excited to work with my Advisory Board colleagues towards a future where all research data can directly contribute to new treatment options for patients in need. The formation of this board speaks volumes to Castor’s commitment to prioritizing the best interests of patients and researchers alike.”
The Advisory Board members include:
- Craig Lipset, Former Head of Clinical Innovation at Pfizer, and Advisor/Board Member at various life sciences startups
- Baljit (Boo) Samra, Strategic Advisor and Consultant, and former COO at Duke Clinical Research Institute. Prior executive leadership positions held at Parexel
- Craig Serra, Global Head of Strategy and Innovation, Data Operations at Novartis. Previous leadership positions at Pfizer and Roche
- Niels van Royen, MD, PhD, Head of the Cardiology Department at Radboud University Medical Center
- Thomas Wurdinger, PhD, Director of the Neuro-Oncology Research Group and Professor at the Amsterdam UMC Cancer Center. Founder of thromboDx (sold to Illumina)
See below for full biographies.
About Castor
Based in Amsterdam, The Netherlands and New Jersey, US, Castor is an international health-tech company founded in 2012 by CEO Derk Arts, MD, PhD to leverage machine readable data to increase clinical trial efficiency. Castor’s Electronic Data Capture (EDC) platform enables every researcher worldwide to easily capture and integrate medical research data from any source in real-time, including clinicians, patients, devices, wearables, and EHR systems.
Castor is currently collaborating with 30,000 researchers, academic institutions and commercial companies across 90 countries to help them accelerate their studies. The platform has supported more than 4,000 studies that cover a broad range of disease areas including diabetes, cardiovascular disease, rare diseases, and cancer. Researchers on the platform generate vast amounts of data from traditional and decentralized trials, and Castor recently reached milestones of 100,000,000 data points and 1,000,000 enrolled patients. As one of the first companies to leverage machine readable data for clinical research, Castor’s platform will enable AI-driven clinical trials and provide invaluable insights to commercial research.
In 2018 Castor raised $6.25m in funding from healthcare investor INKEF Capital in the Netherlands.
Castor Advisory Board
Craig Lipset, MBA
Craig Lipset is a recognized leader at the forefront of innovation in clinical research and medicine development. He is an advisor to technology and biopharmaceutical companies, leading universities, and the venture community, bringing vision and driving action at the intersection of research, digital solutions, and patient engagement. Craig was the Head of Clinical Innovation and Venture Partner at Pfizer, on the founding Operations Committee for TransCelerate Biopharma, and on the founding management teams for two successful startup ventures (Perceptive Informatics and Adnexus Therapeutics). During that time, Craig designed and launched multiple industry firsts. He currently serves on the Board of Directors for the Foundation for Sarcoidosis Research, the MedStar Health Research Institute, and the People-Centered Research Foundation (the central office for PCORnet), as well as on the Editorial Board for Therapeutic Innovation & Regulatory Science.
Baljit (Boo) Samra
Baljit (Boo) Samra has more than 25 years of experience in the clinical research field. He is currently a Strategic Advisor and Consultant providing strategic advice and guidance to Academic Medical Centers, CROs, Biotechnology and Private Equity Firms on clinical trial strategy, planning and execution; mergers and acquisitions; digital health and technologies; organizational structure; operational delivery; quality management; change management. He is also an advisory board member to Biotechnology, AI and Technology Platform organizations.
Baljit is a seasoned leader with global exposure and expertise in IT, quality, operations, strategic account leadership and general management. His experience spans the globe, with international exposure in Europe, the United States, and Asia Pacific including China, Japan, and India.
In his most recent position, Baljit was the Chief Operating Officer (COO) at Duke Clinical Research Institute (DCRI) for three years. Prior to rejoining DCRI, Baljit gained extensive experience with PAREXEL as Corporate Vice President, where he was an integral part of the global executive team.
Craig Serra, MS, MBA
Craig Serra is the Global Head of Strategy and Innovation, Data Operations at Novartis. He was previously the Senior Director and Business Process Owner of Clinical Data Management in Worldwide Research and Development at Pfizer, Inc. While with Pfizer, he was the single point of accountability for oversight of the clinical data management conduct and closeout process area applied across the portfolio of 400+ Pfizer clinical trials, executed by over 10,000 clinical research colleagues, impacting tens of thousands of patients. Prior to Pfizer, Craig was the Director of Data Management and Director of Clinical and Strategic Operations with DSP Clinical Research. At DSP, Craig led biometrics services and oversight of clinical systems and quickly was promoted to additionally drive their business development and revenue generating activities. His previous experience includes project, program, portfolio, and business leadership roles at Pfizer, Roche, Eisai, and Schering Plough. Craig has an extensive and diverse educational background, earning a BA in Psychology from Rutgers University and both an MBA in Management and an MS in Information Systems from Fordham University.
Niels van Royen, MD, PhD
Niels van Royen studied medicine at the University of Amsterdam and received his doctorate degree in 1998. In 2003 he obtained – with honors – his PhD on research in collateral artery. The research was conducted in collaboration with the Max-Planck Institute in Bad Nauheim and the University of Freiburg. Van Royen specialized in Cardiology in AMC Amsterdam (2003-2008). He is currently the Head of the Cardiology Department at Radboud University Medical Center.
Thomas Wurdinger, PhD
Thomas Wurdinger studied molecular biology at VU University in Amsterdam and performed his PhD at Utrecht University. After his postdoc period at Harvard Medical School and Massachusetts General Hospital he now holds a position as Director of the Neuro-Oncology Research Group and Professor at the Amsterdam UMC Cancer Center. His mission is to eliminate late-stage cancers, including brain cancer. His passion for research goes hand in hand with an ambition for entrepreneurship applied to a field with societal importance, e.g. by capturing sequencing data and designing deep learning algorithms to detect cancer from a tube of blood. He strives to translate academic research into clinical applications. This is why he founded two biotech companies, with thromboDx focusing on blood platelet-based diagnostics (acquired by Illumina), and the second being Exbiome BV, which sets out to use microRNAs for diagnostic purposes. He also was one of the first directors of research at GRAIL Inc, a unicorn company aiming to detect cancer early when it can be cured. He is a recipient of the Galenus Research Award and several ERC grants.
For more information about Castor please contact:
Castor:
John Ambrose, Esq
Head of Marketing
[email protected]
Media & IR Enquiries – Optimum Strategic Communications:
Mary Clark/ Supriya Mathur/ Hollie Vile
[email protected]
Tel: +44 (0) 20 3922 1906
 Cherié L. Butts, PhD, Medical Director and Head of Clinical Assessments at Biogen and newly appointed Castor Advisory Board member, commented:
Cherié L. Butts, PhD, Medical Director and Head of Clinical Assessments at Biogen and newly appointed Castor Advisory Board member, commented: