 In order to have a successful clinical trial, researchers must recruit—and retain—patients. This is a struggle that researchers know well; only 1 in 20 patients who respond to a recruitment promotion complete the study, with only 1 in 5 showing up for initial screening.
In order to have a successful clinical trial, researchers must recruit—and retain—patients. This is a struggle that researchers know well; only 1 in 20 patients who respond to a recruitment promotion complete the study, with only 1 in 5 showing up for initial screening.
Many companies are rethinking the way they attract and engage patients in an attempt to optimize outcomes in decentralized clinical trials (DCTs). In a recent webinar, Andy Lipetz, Sr. Director of CRO Partnerships at Castor, Steve Wimmer, Director of Partnerships at 1nHealth and Kamalia Sazali, Senior Product Manager at ObvioHealth, discussed how to better engage patients for better outcomes in DCTs.
About decentralized clinical trials
Historically, traditional clinical trials’ on-site participation requirement has created an access barrier for many who don’t have transportation, childcare, or who otherwise cannot travel to trial sites. The COVID-19 pandemic made it clear that this type of clinical trial was not as sustainable as it was in the past.
“There was a vision for fully decentralized trials, and during the pandemic, it was necessary for patients to participate in a trial from anywhere,” Lipetz explained. “The vast majority of trials now are taking a hybrid or decentralized approach.”
Step 1: Personalize patient recruitment messaging
The first step in patient recruitment is, obviously, making people aware of the existence of any given clinical trial. Raising awareness of the trials is just one step in the process—researchers must also create an experience that patients want to participate in.
Liptez asked Wimmer and Sazali, “How do we do this? How do we encourage more people to participate in clinical trials?”
Both agreed that messaging intentionality was important. Many major pharmaceutical sponsors create well-intended messaging that makes people aware of the need for clinical trial participants, but they’re simply not meeting the potential recruits where they are or speaking to their needs.
“Most people who are on their lunch break on Instagram are not in the right headspace,” Wimmer said. “The messaging for reaching these people needs to be more about them, the patient. What’s in it for them?”
Wimmer continued by suggesting revising ad messaging from a broad approach to a more personalized one that targets people of a certain demographic or with specific conditions.
“There’s not really a demographic that you can’t find using the internet,” he said. “If we’ve managed to deploy a DCT, we have access to the entire population. We can now find the people we need for our studies and ensure we have a representative patient population.”
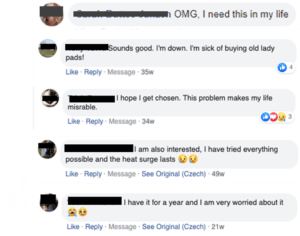 Attracting potential new participants shouldn’t stop at simply running an ad, however. Monitoring the comments on a study ad—and responding to those comments—helps create consumer engagement and raise both awareness and excitement about the clinical trial. When a viewer receives a reciprocal comment on an ad, they realize that there’s a person behind it—not a corporate monolith.
Attracting potential new participants shouldn’t stop at simply running an ad, however. Monitoring the comments on a study ad—and responding to those comments—helps create consumer engagement and raise both awareness and excitement about the clinical trial. When a viewer receives a reciprocal comment on an ad, they realize that there’s a person behind it—not a corporate monolith.
When taking a digital approach to patient recruitment and engagement, it’s important to be inclusive and sensitive to the needs of all age groups. Many advertisers assume elderly populations won’t be reachable by digital channels, or able to access them at all.
“In the US, 75% of the population over the age of 65 is what we would call ‘digitally addressable,’ meaning that they’re using the internet every day, which I think is surprising to some folks,” said Wimmer.
Step 2: Introduce remote components
People are understandably hesitant when it comes to adopting new technology, particularly when it comes to healthcare. That’s why clinical trial companies need to earn the trust of potential participants by making their experience smooth and burden-free.
One immediate way to improve the patient experience is to introduce remote components. When you reduce the number of times that a person has to visit a physical site, especially when they’re elderly, immuno-compromised, or chronically ill, you’re making their clinical trial experience far easier.
“With things becoming more remote,” Sazali said, “study participation becomes more convenient. It means that patients can take part in studies without it being a real inconvenience in their day-to-day lives.”
ObvioHealth recently launched a women’s health study that focused on urinary incontinence. The project was initially a hybrid study, but once the pandemic hit, it had to pivot and go fully virtual.
“The virtual site component was a real game changer. Staff were readily available to patients to answer questions via mechanisms that the patients were already using,” Sazali said.
When it comes to keeping patients engaged, she suggested that offering open chat and phone-call services can help immensely. When patients feel supported and engaged, they tend to want to continue to participate in a project.
“Reducing their burden while allowing them to feel like they’re part of a project helps make this more appealing to a potential patient”, said Sazali.
Step 3: Integrate technology to reduce site burden
 Removing barriers and facilitating access for patients should be the primary focus for researchers as they look to successfully recruit and retain them for clinical trials. But making life easier for study and site teams is important, too. Enrolling and keeping patients engaged in clinical trials is also a huge pain point for researchers. An easier clinical trial experience for patients means that study teams have more time to focus on the outcomes of a trial.
Removing barriers and facilitating access for patients should be the primary focus for researchers as they look to successfully recruit and retain them for clinical trials. But making life easier for study and site teams is important, too. Enrolling and keeping patients engaged in clinical trials is also a huge pain point for researchers. An easier clinical trial experience for patients means that study teams have more time to focus on the outcomes of a trial.
“[Decentralizing clinical trials] helped us resolve issues and questions really quickly. Not having those ad hoc site visits or waiting several weeks to go to a site allowed us to be proactive in supporting patients through their journey,” said Sazali.
Utilizing technologies like eConsent can streamline the experience for everyone involved, from patients to staff. “If you can decentralize your enrollment experience,” Wimmer said, “there’s less burden on-site since everyone who shows up has already consented and enrolled before they arrive.”
When you take a fully digital approach to patient enrollment, you have control over the volume of participants coming to a site. If a clinical trial site is at capacity, you can easily turn off the collection of leads. Researchers also get the benefit of predictability. “We can tell you, with a relative degree of experience, that we can send you 20 to 30 pre-screened and pre-qualified leads per week. All you have to do is call them and schedule appointments,” Wimmer said of 1nHealth’s technology.
For both participants and researchers, communication has historically been a pain point—it can be difficult for patients to get in touch with clinical staff, and vice versa. Some worry that because clinical trial participants have increased access to staff, these technologies will actually increase the burden although Sazali hasn’t seen this trend. “It’s about being there to answer a quick question about the study or about the responsibilities that the patient has. Opening those lines of communication allows questions to be answered efficiently, sometimes through a chat, without having to schedule an ad hoc visit or a phone call.”
One major benefit of decentralized clinical trials is the sheer amount of data that becomes available to researchers. With DCTs, patients typically wear monitoring devices or take measurements themselves at home. Researchers can take that data and input it directly into the study database for remote monitoring.
“Instead of those point-in-time, at-site readings, we’re getting regular at-home readings or in some cases, continuous data,” Sazali said.
These regular readings give clinicians a more accurate picture of the study journey and how their patients are doing at home. It also gives access to an immense amount of data that wouldn’t be captured at a traditional trial site.
Step 4: Build an automated workflow
How does one go about picking the right tools for a clinical trial to ensure their processes are streamlined and integrated the way they need to be?
The panel agreed that a lot of it has to do with trial and error. Providing training on new technology that’s easy to understand and accessible can make a massive difference when someone’s deciding whether or not they want to participate in a clinical trial. It’s important to focus on creating technology solutions that actually reduce patient barriers and burdens, not compound them.
“I think the best way to create a streamlined experience for patients is to automate everything,” Wimmer added. “This might look like an online pre-screener that goes into a chat bot for follow up or automated scheduling. We must figure out how to make new tech play nice with as many systems as possible.”
Developing an automated workflow not only improves the patient experience, but is proven to deliver better outcomes, as demonstrated by the COVID-RED (Remote Early Detection Study).
This Julius Clinical and Takeda-supported decentralized trial uses an app paired with the AVA wearable to monitor changes in vitals, attempting to alert participants to possible COVID infection before they show symptoms. As a fully-remote study, participants needed to be engaged and retained remotely, while data was automatically collected from thousands of devices.
Using API connection, researchers were able to create an automated workflow that connected eConsent and EDC technology to allow for timely data insights and clear trial oversight. The result? Julius Clinical succeeded in recruiting over 17,824 participants in 15 weeks during the COVID-19 pandemic.
Step 5: Prioritize the patient
 Finally, the team agreed that one of the most important things to remember is to not lose sight of who these technologies are really for—people.
Finally, the team agreed that one of the most important things to remember is to not lose sight of who these technologies are really for—people.
“Yes, we have access to all these really great, amazing technologies, but it’s really important to not lose sight of the human component in all of this”, said Wimmer.
Imagine you are a patient participating in your first clinical trial. What factors encouraged you to join? What aspects would be burdensome? How would technology ease that burden? It’s only when we are able to fully understand the patient experience that we can begin to realize the potential of decentralized clinical research.
For more on this topic, watch the webinar Castor held with 1nHealth and ObvioHealth to hear thoughts on keeping patients engaged in today’s modern clinical trials.