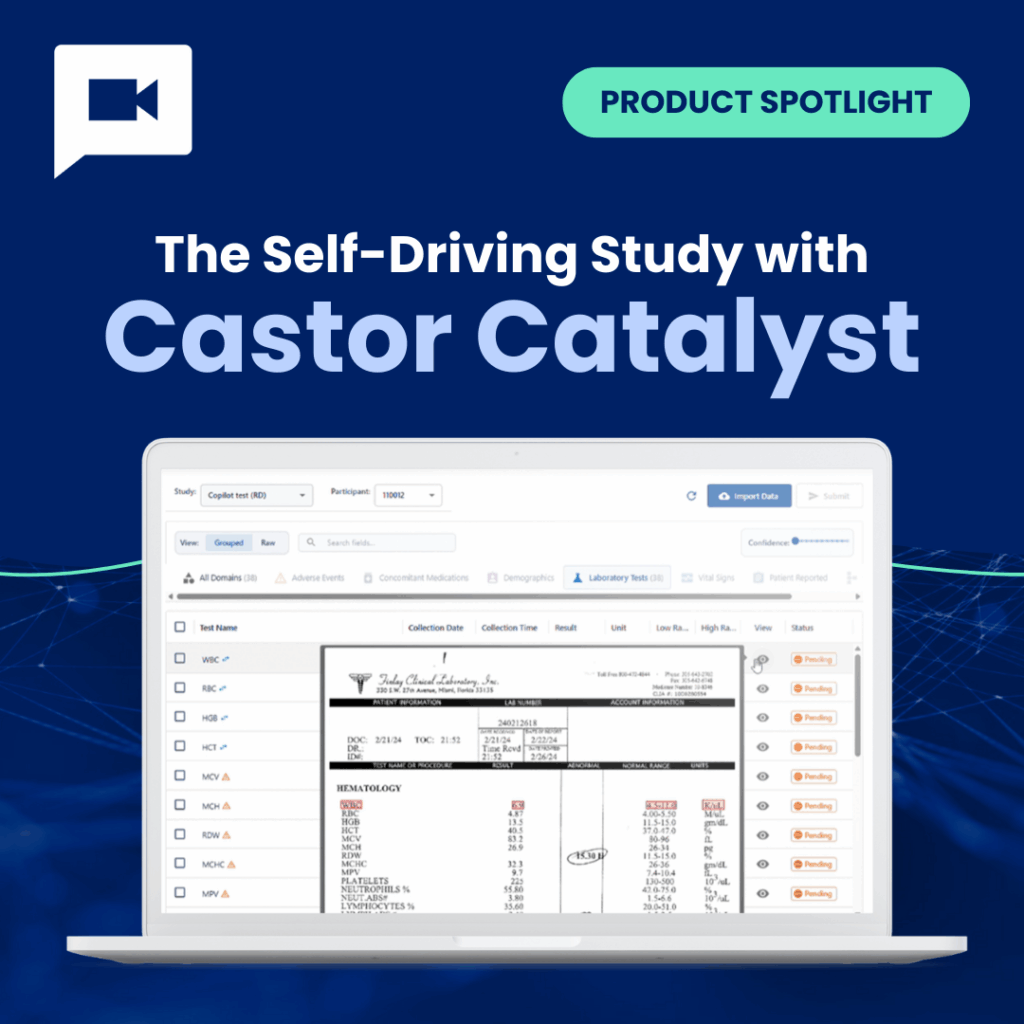
In the summer of 2011, during my final medical internship at the Intensive Care Unit in Alkmaar, the Netherlands, I was asked to support a clinical study. The data collection setup? Dozens of individual Excel files — one per patient — with the plan to merge them manually at the end of the study.
It was immediately clear to me: this was not sustainable.

Having spent six years as a freelance PHP developer building medical applications, I knew we could do better. I started coding a solution that would allow clinicians and researchers to collect and manage their data more efficiently and accurately.
After just two weeks of development, The Research Data Collector was born. We piloted it in the ICU, and quickly saw the potential. What surprised me most was how common this problem was. Across hospitals and research centers, investigators were stuck between outdated tools like Excel or SPSS, costly commercial systems, or open-source software that required too much technical lift.
That gap became my opportunity.

A few months later, a friend beginning his PhD in Amsterdam became our first customer. Using Excel templates to define structures, I built the first real iteration of what would become Castor EDC. Soon, word spread. I landed my second and third customers through my friend’s network, and for the first time, Castor began generating real revenue. While balancing my PhD at the Academic Medical Center, I invested every spare hour into improving the system.
As demand grew, I took a leap of faith: we pooled resources and hired our first two developers. We completely rebuilt the application — what I consider “Version 2” of Castor. At the time, we were thrilled to have a handful of customers and a few active studies. I could never have imagined that, just over a decade later, Castor would support more than 15,000 active studies across 90+ countries.
The turning point came in 2012, when I met Sebastiaan, our Lead Developer. Together, we built “Version 3,” the foundation of the platform that today powers clinical trials for some of the world’s largest CROs, biopharma companies, and research institutions.
What began as a small project to fix a local problem has scaled into a global movement.
- 15,000+ active studies
- 90+ countries
- 250+ employees driving innovation
- 98% customer satisfaction rate
- 300+ COVID-19 studies supported at no cost
- Proud partner of WHO’s Solidarity Trial
Today, Castor is much more than a data capture tool. We are a strategic partner for the future of clinical research, helping our customers accelerate trials, reduce operational burden, and unlock the full potential of their data.
Our mission remains the same: to make clinical research faster, smarter, and more accessible for all. And while the numbers have grown, our passion for solving real problems at the ground level remains unchanged.
What started with a few patients in an ICU is now transforming clinical trials worldwide. And we’re just getting started.
— Dr. Derk Arts, Founder & CEO, Castor