The path to market for medical devices can be fraught with obstacles. One of the first challenges confronted by medical device manufacturers is setting up the preclinical research phase to demonstrate that the technical characteristics of the device meet safety and biocompatibility standards. This phase is otherwise referred to as the prototype stage; verification and validation; an early version; or not-for-human use. At this point, device prototypes are tested in controlled laboratory settings using in vitro (eg. in a test tube) and in vivo (eg. in a human) methods.
During the preclinical phase, research and development (R&D) scientists gather critical information about the product’s potential use for people in order to test those relevant characteristics. Their methods must adhere to Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP), the regulatory standards that define the minimum requirements for planning, conducting, and reporting nonclinical safety studies.
Originally, GLPs were written primarily to apply to nonclinical studies of chemicals and pharmaceuticals, but they are now being applied to medical device studies too. GLP regulations have been updated to reflect this expanded scope, where previously the industry had to employ its best judgment as to how its language corresponds to medical device studies. Regardless, the goal of any preclinical phase is to gather sufficient data for analysis in order to move the device along the path to market.
Challenges in preclinical research
The success of the crucial preclinical phase hinges on the collection and aggregation of data. One of the initial challenges in setting up a smart data collection strategy is that R&D teams are made of individuals coming from different backgrounds and approaches. Invariably, there are growing pains as a newly assembled team learns how to work together efficiently.
There is also the procrastination factor. Too often, a preclinical phase employs a haphazard approach to data collection with a piecemeal approach of spreadsheets, text documents, email, and paper documents being used. The intention is to transfer over all of the records into a digital format later. This is a problem for two reasons: 1) each time the data is touched or re-transcribed, there is an increased risk of error and 2) a casual approach will not pass muster with the GMP.
Even if a team is determined to kick things off with a single digital system for their preclinical phase, they can be stymied by inefficient data collection tools. The main issue is usually that data collection tools are built to revolve around patients. This is often not practical in a nonclinical setting which usually focuses instead on bench testing or animal and cadaver testing. Fortunately, there are great solutions available which we’ll dive into next.
Use an Electronic Data Capture (EDC) system from the start
The old‑fashioned sentiment of “begin as you mean to go on” can prove extraordinarily helpful in the preclinical phase. Collect all data digitally from the start — your stakeholders will thank you later! It can be tempting to start off with paper, intending to transfer everything over into a digital system later; however, this is not the best choice, as we’ll explain.
Of course, getting to market quickly is the goal of any medical device developer. Aligning your processes at the beginning of a project will help make this happen. Creating an effective database from the get-go will make for a smooth transition from the development phases. When a project moves through the phases into clinical trials, it is enormously helpful to already have an EDC set up.
Start by acquainting your entire team with your chosen EDC from the start. This will enable them to focus on iterating the product development. Take advantage of the breathing room at the beginning of a preclinical trial to set up all your processes and systems. When the stress of a large late-phase clinical trial hits, having smart strategies already in motion from the preclinical phase will prove to be a huge relief. If you have the right forms set up to capture data, a secure and compliant system to store it in, and formulas set up for various calculations, you’ll be well set up for success.
Start with this checklist to get started:
- Hook up devices with connectivity abilities to the EDC right away
- Integrate Laboratory Information Management System (LIMS) into the EDC to ensure data integrity across systems and create efficiency across teams.
- Automate as much as possible (much more on that below!)
- Import any data already collected, including large multimedia files
- Export and import your study structure
Ditch old-school tools

In a clinical study, relevant clinical information on study participants is collected using Case Report Forms (CRFs). When the study is completed, the CRF documents are gathered and sent to the sponsor. In the past, CRFs used to simply be paper forms, requiring mountains of paperwork and lots of manual work as the data needed entered into a statistical analysis tool. In some cases, this paperwork must then be stored for 15+ years!
The modern approach to CRFs is building custom electronic versions to be filled out on a web-based platform within an electronic data capture (EDC) system. The data collected is stored directly in a central database.
Sometimes teams ditch the paperwork and use general purpose applications such as Excel, Google Forms, Access, and SPSS to collect data. A warning though: these were not developed as data capture & collection tools specifically for healthcare and are not suitable for tracking data changes and errors. They may also result in data leakages, such as incorrect transfer of sensitive health information, which can result in fines. For these reasons, such applications are not compliant with relevant legislation.
Instead, embrace modern tools. Not only will it allow research professionals to work faster, it demonstrates regulatory compliance and sets the rest of the project up for success. Carry over forms from the first prototype all the way to human trials.
Demonstrate compliance from the get-go
 If speedy regulatory approval is the goal, then demonstrating regulatory compliance from the start is a must. One of the ways to do this is by using a validated EDC with GCP/GLP compliance.
If speedy regulatory approval is the goal, then demonstrating regulatory compliance from the start is a must. One of the ways to do this is by using a validated EDC with GCP/GLP compliance.
Demonstrate that the research and development processes meet all the compliance and audit requirements by using EDC features such as:
- Audit trail, data trail, and edit trail: All actions that can be carried out in Castor are saved in the audit trail. Granular audit logging enables you to review all edits and changes in the study structure, data collection, and study management. Data can only be archived, not deleted, in line with GCP.
- Personalized accounts: Each user requires their own individual account. Sharing of passwords should not be permitted and strong password choices should be used when creating or changing passwords. SSL should be used for log-in and account information encrypted.
- No unauthorized data access: This allows for reconstruction of which person possessed which rights when. An up-to-date user rights overview should be available in the system, and all alterations of user rights logged in the audit trail.
- Access management: The study admin is in charge of authorizing access to data, which should happen on an individual basis. The study admin is responsible for inviting everyone involved in the study and giving them proper rights, ensuring that study data cannot be accessed by unauthorized people.
- Safeguarded randomization: The randomization blinding must be safeguarded. Only users with randomization rights should be able to randomize, and only users with randomization view rights should see which group a subject ended up in.
- Reason for changes: An explicit reason for each data change should be logged when any change is made in a CRF.
- Guaranteed data storage: All sensitive data should be stored on servers hosted by certified hosting parties.
- Backup: Backups should automatically occur several times per day and be moved to a separate geographic location daily.
Tailor the database
The bad news is that a one-size-fits-all database rarely works well. The good news is that it’s possible to save a large amount of time by tailoring a database to a trial’s specific needs.
One way Castor offers outstanding flexibility is through the “Records” option. It can be used to track anything: a medical device, an animal or a human subject. It is possible to search, create, export, import, print and filter the records.
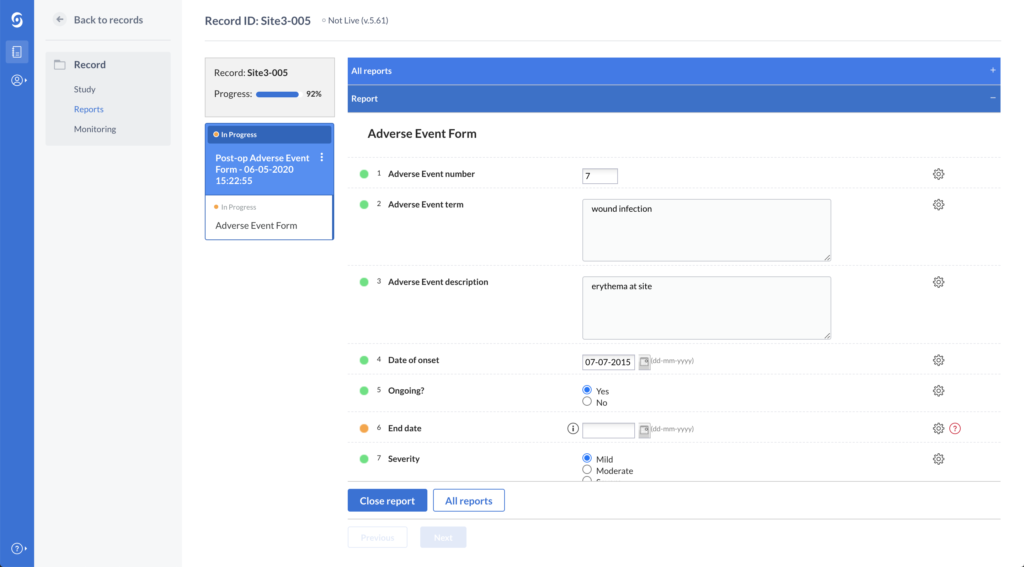
In Castor it is also possible to create unscheduled forms through the “Reports” functionality to store additional information as needed. Reports seamlessly record any protocol deviations (eg. low number of daily measurements or incorrect reference data) in real-time, making it easy to ensure that a trial is following the study protocol.
The scope and methods used for preclinical data collection can vary greatly depending on the kind of device under development and the preclinical phase’s setup. A great EDC allows users to put everything into the database, so that there are no more side note papers with ‘other data’. Everything should fit neatly into one platform.
Automate, automate, automate
Another key in building a comprehensive database is automating certain events and tasks. At Castor, we believe that anything that can be automated, should be automated! It saves countless hours and decreases risks of errors. That’s why we’ve created built-in data quality-boosting features such as:
- Edit checks: These advanced data validations enable research professionals to add dependencies and validations to the forms prior to data collection, significantly improving data quality. Setting data limits for all captured data reduces human errors and ensures accuracy. It also flags errors or results falling out of predetermined parameters.
- Calculation Fields: Automatically calculate various scores relevant to your study by setting up complex data dependencies (field logic) and data validations (edit checks). Automating mathematical calculations speeds a trial along and omits manual work.
- Monitoring functionality: Offers a complete overview of all the queries, data validations, and dropped verifications. Users can easily filter validation fields in their study forms, reports, or surveys by Exclusion, Warning, and Message.
- Survey Triggers: Increase overall participant enrollment and retention by automating survey events. Castor’s automation engine works with both action and time-based triggers to send relevant surveys at the right time.
The bottom line? Automating study operations and actions increases data quality and efficiency, while reducing manual labor.
Provide stakeholders with peace-of-mind
Accountability starts right at the beginning of the medical device development process. Long before device manufacturers are accountable before notified bodies, R&D professionals need to provide regular updates to other stakeholders. While this is necessary, it can also be time-consuming. There is an expectation of regular updates, but these can cost valuable time and take away from the team’s momentum.
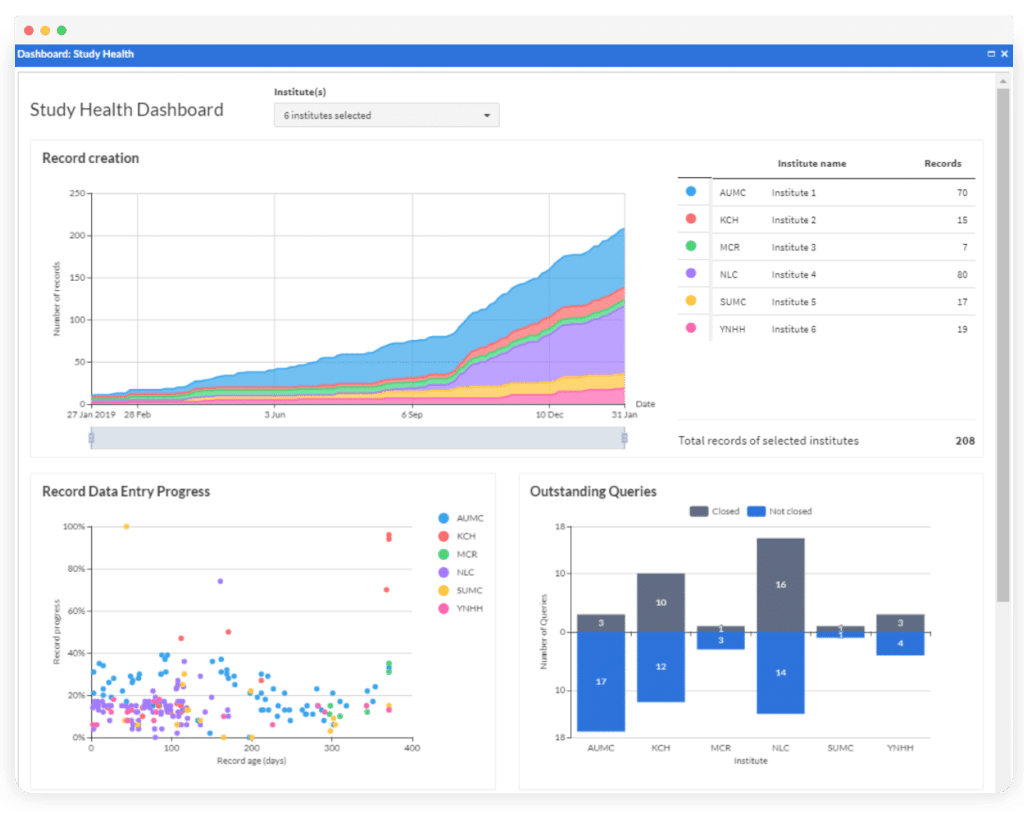
With Castor, it is possible to provide limited access to the study to allow other stakeholders to track progress. This greatly reduces the amount of work involved in keeping stakeholders abreast of progress. Users can use Castor’s Study Health Dashboard to provide a graphical representation of study progress including:
- Participant recruitment
- Monitoring activities
- Data collection progress
When sharing forms with stakeholders, it’s crucial to control what they can access and see. In Castor, it is possible to hide certain parts of the form from specific users. For example, it is possible to give access to view and export Adverse Event data only, while hiding regular data if necessary.
As creators of an electronic data capture system, our team at Castor understands that software license fees can add up quickly. We also understand the pressure to conduct preclinical studies before you can progress to the next phase of medical device development. That’s why we offer affordable bundle pricing tailored to your exact needs. We even have special pricing for startups, because we’ve been there ourselves!
Do you have a preclinical phase starting? Let’s chat about how we can simplify the process for you.


