Despite the global trend towards cloud-based data services, many biopharmaceutical and medical devices companies continue to collect clinical trial Case Report Forms (CRFs) on paper. Although cloud-based Electronic Data Capture (EDC) systems for eCRFs have been around for over 15 years, it is estimated that between 25 and 50% of new clinical studies still rely on paper.

According to a survey of biopharma executives, the main obstacles to using an EDC system are its up-front costs and security concerns. In addition, respondents cited the additional training required for personnel and the limited size of their clinical trial as arguments why they would not opt for an EDC system.
We argue that these obstacles are largely based on myths. In this article we will address the 4 largest myths of the benefits of paper-based CRF.
With drug development costs becoming higher, and medical device clinical regulations becoming more stringent, there is a strong need to reduce the cost of clinical trials. If you do not yet use an EDC system, we will aim to convince you that an EDC makes sense for your company.
Myth 1: Paper-based studies are less expensive
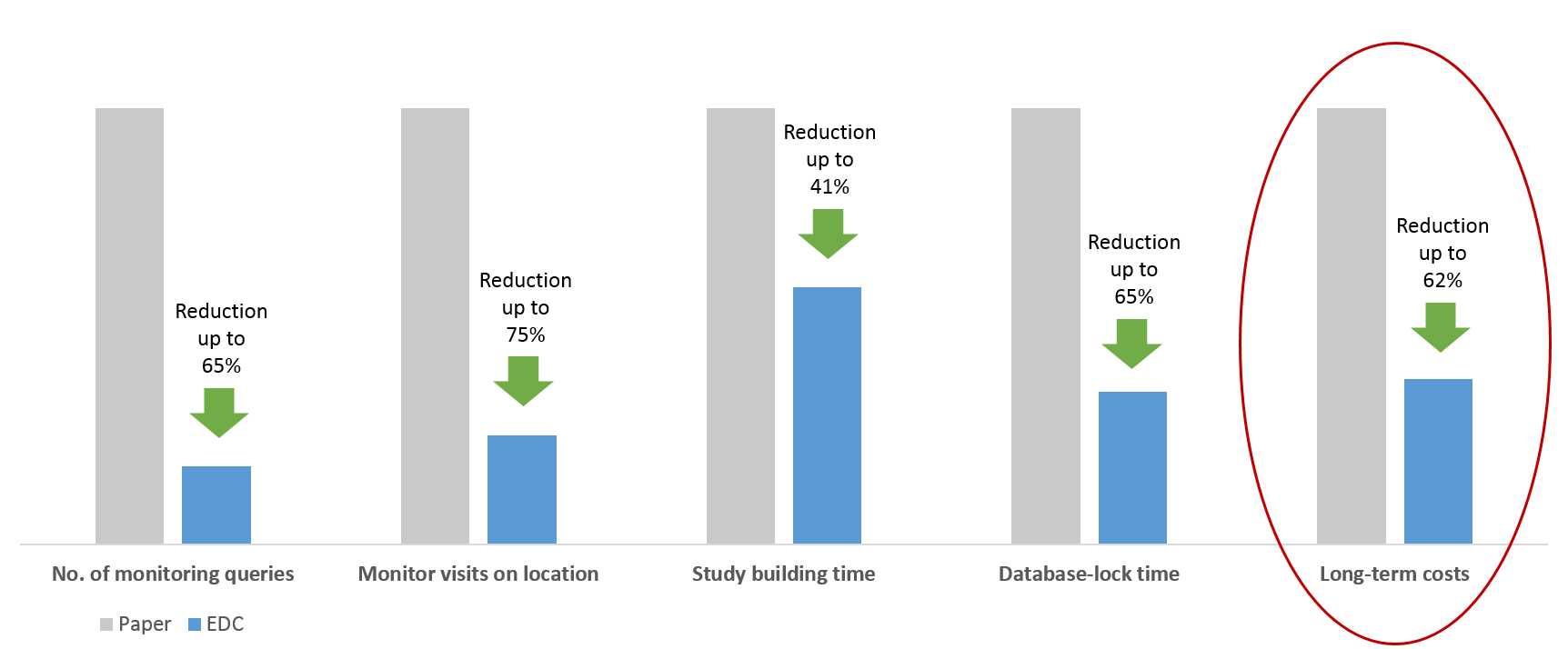
It is true, running a clinical study on paper does not require monthly license fees. The low upfront costs of starting a clinical study using paper alone is tempting. However, what seems inexpensive in the short term is actually more costly in the long run. Pavlovic, et al. estimate that when comparing paper with electronic case report forms, the long-term costs of using paper is 49-62% higher. This is substantial, to say the least. The primary contributor to the cost is the high error rate that follows from manually re-entering data collected.
Using an electronic case report form (eCRF), on the other hand, allows for the data to be entered directly into the system. It also allows for automatic data validations, which significantly reduces the potential for errors in the data. This will significantly reduce the time monitors have to spend on location reviewing data. According to a study by Banik et al. collecting data using an EDC leads to 82% less monitoring queries raised when compared with paper. In addition monitor site visits may be eliminated by 75%.
Myth 2: Clinical trial timelines cannot be reduced using software
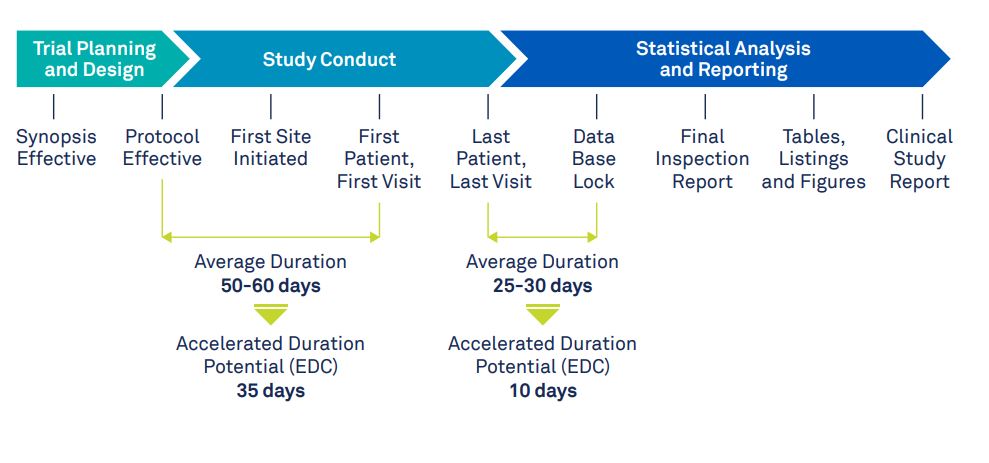
While you would expect that clinical trial timelines are unaffected by the method of data collection, it has been shown that using an EDC can reduce trial timelines. According to this white paper, study-building and database-lock times can be reduced by 27-41% and 41-65% respectively. During the study-building phase, the time-saving benefits come primarily from reusing existing eCRFs and study setups rather than building them from scratch. After the last patient visit, before database-lock, the data is checked for completeness by the data management team. All queries are resolved and the data is cleaned. This process leaves the statistical analysis team waiting, wasting valuable time. An EDC system on the other hand provides a clear overview of any missing data and unresolved queries. This allows management to quickly take action and electronically sign the verified record before database-lock.
Myth 3: Switching to an EDC and eCRFs will require significant training
Yes it is true, paper CRFs does not require any software training. Switching to an EDC system therefore seems like a significant time investment. However, the actual time required to train your personnel for using an EDC system is limited. While some traditional EDC systems require highly-skilled technical staff, more modern EDC systems such as Castor EDC have been built for user friendliness, automating features like User Acceptance Testing (UAT) in clinical trials. New organizations starting a clinical trial in Castor EDC require an average of 1-3 hours training. Clinicians entering data save time and typically require very little formal training. Castor EDC also offers premium support modules and study building services that reduce the burden on your organization. Castor EDC partners with clinical CRO ‘s such as Julius Clinical for additional clinical services such as study building.
Myth 4: Clinical data stored on paper is more secure
One seeming benefit of storing clinical trial data on paper is that it can be securely locked away from outside access. Paper can indeed be stored in a physical vault, but in practice its use is often far less secure than data stored in the cloud. Documents often lie around on staff desks, or end up in a briefcase. Documents also need to be physically transferred to a central location, which poses a security risk. Using a cloud-based EDC system mitigates these risks by using military-grade data security (ISO 27001, HIPAA), off-site backups, and transferring data using SSL encryption. Confidentiality is safe-guarded through flexible user management and an audit-trail. These features also enable your company to meet GCP compliance requirements for best clinical practice.
Conclusion
Overall, it is clear that the benefits of using paper over EDC do not hold up in the long run. With clinical trials becoming more expensive, companies should aim to streamline as many processes as possible. Using an EDC for collecting and storing clinical data therefore seems to be a simple choice. In particular:
- Using an EDC system and eCRFs can specifically lower clinical trial costs by improving data quality and reducing monitoring costs.
- Trial timelines can be shortened by reducing study-building and database-lock times.
- Using a system such as Castor EDC does not require a significant time investment in formal training.
- A cloud-based EDC system mitigates many security risks associated with physical paper and enables your study to become GCP compliant.
Castor is a user-friendly and affordable EDC system used by academic researchers and biotech & pharma companies for clinical trials.