Patient reported outcome measures in clinical trials have traditionally been done on paper. Surveys are a common way to collect data from study participants. Surveys are questionnaires that allow data to be collected from a predefined sample in a population [1].
What are patient reported outcome measures?
Patient reported outcome measures, or PROMs, are an easy method for measuring a patient’s health status or health-related quality of life. These capture data from moments in time through medical questionnaires which patients complete independently [2].
By filling in the questionnaires, patients directly report on how their symptoms, daily functioning and general well-being are perceived during the study. Therefore, PROMs ensure to record not only the researcher’s observations and interpretation but the patients’ perspective on their own health.
Why are patient reported outcomes important?
The broad goal of clinical trials is to improve healthcare and its outcome for the population. By collecting patient reported outcome data, researchers get a brief insight into the frequency and variety of symptoms as well as the disease’s actual impact on daily life. These findings can later be used to close the gap between clinical research and therapy to ensure a patient-centered and high-quality care practice
In the past, surveys have been administered on paper, which requires tedious administration and logistics, and can also pose a private health information security risk. Thanks to advancement in digital technology, it is now possible for researchers to easily collect data electronically and in a secure way using tools like ePROs (electronic Patient Reported Outcomes) or eCOA. Patients can complete secure electronic surveys sent via email, saving time, increasing engagement, and requiring less administration. At the moment, more than 26% of studies in Castor are using surveys.
Benefits of eCOA / ePRO
-
Electronic medical questionnaires are easy to distribute:
A major benefit is a more efficient and streamlined workflow, equating to time saved for researchers and participants. Often, for example, travel time to the clinic for data collection can be a barrier for participants and negatively impact the study, especially when researching rare diseases or small gene pools [3].However researchers should use tools designed and built for medical research, both for security and data compliance.
-
Electronic Surveys are cost effective, requiring minimal research power to reach people and collect data.
With the correct electronic data capture (EDC) tool, researchers can send patient questionnaires directly from the system and do not need to import or copy data from paper. Well designed surveys will collect high quality relevant research data, but require careful crafting and evaluation of wording and questions [4].
As discussed above, medical questionnaires need to be well crafted to ensure they are valid and reliable. It is also important to ensure that the correct population sample is selected. As with all study designs, surveys can introduce bias as a result of poor responses or no-responses (researchers’ cognitive bias).
How to create good clinical outcome assessments
As researchers, the challenging task lies in creating a well designed patient questionnaire that measures what it claims to measure ensuring that it is valid. External validity is important for the generalizability of the study, ie. are the inclusion or exclusion criteria properly defined, can the results be applied to a population [4]. And internal validity is related to the robustness of the study, ie. does it have sufficient statistical power, proper control groups, randomization and blinding necessary for clinical trial research [4]. And a reliable questionnaire that will produce consistent results upon repetition [1].
When generating a patient questionnaire, the questions can be close-ended or open-ended. With close-ended questions, researchers set the range of answers on a scale or a range of tick-boxes [1]. Open-ended questions or free text can enrich quantitative data, and researchers will want to plan in advance how this data will be analyzed [1].
Standardized questionnaires can also be used, see an example below of an EQ-5D Questionnaire from Kieran Bond of Aridhia [2]. These widely used forms ensure that a high level of validity and reliability is achieved throughout the research.
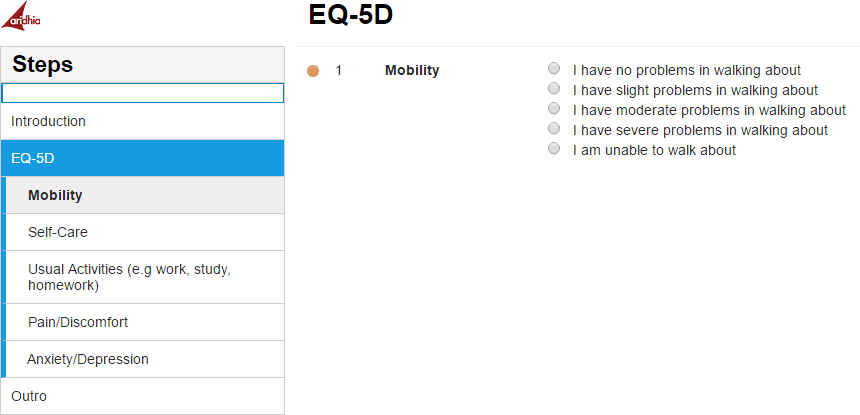
These widely used forms ensure that a high level of validity and reliability is achieved throughout the research.
Using Castor eCOA / ePRO to send medical questionnaires to patients
With Castor eCOA / ePRO you can create complex surveys in minutes, using more than 21 field types, pre-built templates, and validations. You can also reduce time spent on rebuilding surveys from scratch by reusing existing surveys.
You can choose from tried and tested electronic surveys shared by Castor users in the Castor Form Exchange. Standardized forms, such as the 36-Item Short Form Health Survey (SF-36) that measures quality of life, can be easily downloaded and re-used.
By using Castor’s automation engine you can increase participant enrollment, retention, and experience through automated patient engagement. You can also easily manage survey participants through bulk invites, automatic triggers, and a dynamic dashboard.
Researchers can schedule surveys and create emailing schedules to distribute patient questionnaires on certain dates or according to a custom timeline.
Using encrypted email addresses, clinical data entry is combined with outbound survey invitations sent to study participants. And at the push of a button, researchers can send a clinical outcome assessment to hundreds of participants, monitor its status and see results directly in the study dashboard.
Check out our webinar on how to build surveys in Castor eCOA / ePRO.
Sources:
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC420179/
- http://www.aridhia.com/blog/building-trust-and-improving-participation-in-clinical-trials-using-innovative-electronic-data-capture-platforms/
- http://www.bmj.com/content/350/bmj.g7818
- http://www.bmj.com/about-bmj/resources-authors/bmj-right-journal-my-research-article


