With the recent announcement of the formation of a new organisation, NHSX, to oversee digital presence, data, and technology, the UK Government has put the onus on the NHS to embrace technological innovation. The Government, alongside NHS England, has long been striving to increase the speed of digital evolution within the NHS. Much of the data-centric technology currently used within the NHS is outdated and does not enable trusts to quickly and easily share data, which can lead to delays and difficulties in providing care.
With this in mind, the NHS’ long term plan is now largely focused on implementing strategies to increase the uptake of cutting-edge technology to benefit NHS trusts and patients across the UK. The formation of NHSX serves to reinforce the importance of achieving full implementation of these technologies. Whether NHSX will be able to achieve their goals, only time will tell.
Here at Castor, one of the questions we have been looking into is ‘How will the formation of NHSX impact medical research in the UK?’
What is NHSX’s remit?
The UK Government has listed a wide range of responsibilities that NHSX will be taking on, which can be found here. NHSX will have full remit to take over the NHS digital strategy and accelerate the Government’s vision for technology in healthcare, and so will be in a unique position to ensure that NHS trusts procure the best technology on the market. Furthermore, NHSX will have the opportunity and obligation to look at big picture items such as Electronic Health Record (EHR) adoption, as well as a wider range of digital developments, applications and cloud solutions for NHS staff and providers.

How can NHSX support medical research in the UK?
As the full remit of NHSX is revealed, it becomes clear that there are a number of positive ways in which the organisation can support medical researchers across the UK. UK medical research and data use are likely to be particularly impacted by NHSX’s input in the following four ways:
- Developing best practices for improving data-sharing and transparency across the NHS.
- Setting national strategy and mandating cyber security standards.
- Ensuring that NHS systems integrate with each other across the NHS and allied service providers.
- Reforming procurement: helping the NHS choose the right technology.
Data-sharing and transparency across the NHS
Data sharing in medical research has long been the subject of debate, and the FAIR data principles (2016) were established to guide improvements to research infrastructure. The proponents of FAIR assert that all research data should be Findable, Accessible, Interoperable and Reusable to facilitate collaborative and retrospective research, bring medical innovation fully into the digital age, and ultimately improve the quality of future healthcare.
These FAIR principles are wholly reflected in Castor’s goal of helping ‘accelerate medical research by unlocking the potential of every byte of research data.’ Castor’s founder, Derk Arts, MD, PhD, elaborates on this in his article about Castor’s commitment to enabling scalable FAIR data for all medical research.
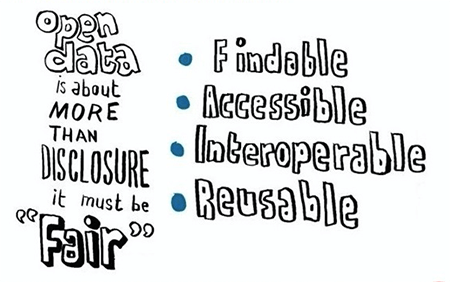
Source: Scientific Data
The aim of FAIR data in practice is to enable research institutes around the globe to maximise the value of each datapoint gathered in research. With innovative and intuitive electronic data capture technology, it becomes possible to quickly and easily combine all types of clinical trial data, whether from NHS trusts, dedicated clinical trial units or private research organisations, ensuring accurate, securely accessible data. Easily accessible, FAIR data can help inform research and provide verifiable, timely results to guide treatment options, for example for early-onset dementia in the UK/worldwide. The value of being able to see a verified combined data set, rather than manually collating studies using a wide range of data collection methods is clear. The use of seamless, synchronised data capture technology will dramatically increase the likelihood and speed with which this research data can be transformed into actionable knowledge that can benefit our healthcare systems and patient care.
The creation of NHSX means there that the issues of data quality, sharing and reusability in medical research will be at the forefront of focus and future mandates. The FAIR data principles will inform the uptake of new technology, and NHSX can help to make the UK a world leader in secure global data access and reuse.
Cyber security standards
Previously, NHS Digital published clear cybersecurity and data protection guidelines for healthcare organisations, with particular regard to cloud-hosted solutions and remote access. This was of critical importance, as any organisation that had access to UK NHS patient data needed to provide assurances that they practice impeccable information governance. In particular, local and national NHS guidelines followed various frameworks and guidelines, including:
- Health and Social Care Network Certified Hosting Partners
- ISO27001 – Best Practice Framework for security
- Information Governance Standard Framework
- Information Governance Toolkit (IG Toolkit)
- The Caldicott Principles
- Good Clinical Practice (GCP) guidelines
- General Data Protection Regulation (GDPR)
As data within the NHS moves into the remit of NHSX, NHSX will be obliged to provide healthcare organisations and suppliers alike with clear guidelines for future cybersecurity standards. This will, in turn, clarify mandatory data protection standards and legislation, and help ensure that patient data is protected at all times.
Castor provides an ISO27001, ISO9001, and GDPR compliant cloud infrastructure. We are fully compliant with Good Clinical Practice (GCP) and 21 CFR Part 11 and we employ stringent security procedures to ensure we protect our customers’ data in the best possible way.

System Interoperability
Across healthcare organisations globally there has been a strong drive to improve the interoperability of disparate clinical systems. However, much more still needs to be done in this area. All too often, hospitals are limited by having multiple clinical systems for different tasks that don’t effectively integrate or talk to each other. This can cause huge practical problems, such as:
- Slow, inefficient processes with multiple clinical systems required by clinicians to find the patient information they need.
- Manual data re-entry into clinical systems which could have been automated if the systems spoke to each other.
- Governance issues and a much more difficult IT landscape to manage.
Based on their given remit and responsibilities, it’s vitally important that NHSX uses its platform to create a coherent digital policy that embraces change, improves access to digital solutions and ensures seamless interoperability between providers. Complying with the updated HL7 FHIR standards will be a key focus, as healthcare providers will have a responsibility to use interoperable solutions, helping to provide the best possible quality of care.
At Castor, we are supporting interoperability by making it easier to conduct multicentre trials across universities, hospitals and other research centres, and connecting the data to EHR systems. One of the ways in which we do this is through our HL7 FHIR Importer tool which helps to ensure researchers do not have to manually re-enter patient information from the EHR.
Reforming Procurement
Traditionally, NHS procurement is a complicated, time-consuming and difficult process. Often there is a lack of clarity regarding the process and unfortunately, it can be very difficult for NHS trusts to procure the right technology even in situations where they know exactly what they want. As cost pressures have risen over recent years, a greater emphasis has been placed on the price of the solution which has sometimes been at the expense of the quality.
At Castor, we have seen first-hand the challenges that researchers face when wanting to purchase a cloud service. In terms of the electronic data capture market, this can lead to a situation where the researcher has either:
- An inefficient and unsuitable solution for capturing, recording and measuring data (i.e. Excel or paper-based); or
- An expensive electronic data capture solution that is costing their department or institution a significant portion of their annual budget.
This is therefore a vital area that NHSX needs to reform. By simplifying the buying process, removing some of the red tape associated with NHS procurement and speeding up the decision-making process, NHSX can enable trusts to have faster access to a wide range of invaluable digital solutions. The NHS can then start to reap the benefits of innovative, world-class technologies.
Castor is directly supporting these innovations within the NHS and the medical research community, by providing a user-friendly, compliant and affordable data management solution.

Conclusion
Upon the formation of NHSX, Health Secretary Matt Hancock said: ‘This is just the beginning of the tech revolution, building on our Long Term Plan to create a predictive, preventative and unrivalled NHS.’ NHSX has been created with an ambitious goal: to ensure that NHS trusts adopt the very best in digital technology. If they succeed, this will herald a new age of digital solutions for medical research, revolutionising clinical trials and the capture, storage, and management of data. NHSX can elevate the UK to the global arena of data sharing and interoperability so that we see more actionable knowledge benefitting our health system from the data collected on these shores and beyond.
Here at Castor, we are working closely with various UK institutions and hospitals to improve the quality and reusability of the research data that they are collecting. Furthermore, we are working hard to become a pioneering player in the field of Open Science and to enable researchers to share their data between research projects worldwide in accordance with FAIR data principles. We believe we can help maximise research impact, and are striving to achieve this in partnership with the community of researchers using Castor both in the UK and across the globe.


