Under the new Medical Device Regulation (MDR), companies planning to certify (or re-certify) their medical devices will face a plethora of new regulations and requirements.

One significant change that has faced controversy is the Post-Market Clinical Follow-up (PMCF) clinical data requirements. The MDR’s requirements help manufacturers to establish continued safety of their devices. High-quality data (such as data captured via EDC, ePRO, Randomized Control Trials, etc) provides insight into the safety and performance of the devices after they are on the market. Data collected during PMCF helps identify the device usage problems and clinical outcomes.
While the MDR has substantially increased data requirements, PMCF has the potential to bring a lot of value to medical device companies. This blog discusses 3 key benefits of PMCF for your company.
But first, let’s quickly discuss the goal and scope of PMCF.
What is the goal of PMCF?
Under the MDR, PMCF will be enforced as an integral component of Post-Market Surveillance (PMS) activities. During PMS, companies collect and review the experiences gained from their devices throughout their lifecycle. The new PMCF clinical data requirements will help track the performance of devices even after they’re on the market to ensure continued compliance. The data collected during PMCF will provide insight into developing the device’s design, thus creating a continuous cycle of feedback to help improve device design, user experience, and quality management.
Although a reactive approach was usually considered sufficient under the Medical Device Directive (MDD), the MDR mandates that processes should be explicitly ‘proactive and systematic’. By far the most proactive aspect of PMS will be the collection of clinical data during PMCF. To quote the official MDR Regulation 2017/745, PMCF should aim to:
(a) confirm the safety and performance of the device throughout its expected lifetime;
(b) identify previously unknown side-effects and monitor the identified side-effects and contraindications;
(c) identify and analyze emergent risks on the basis of factual evidence;
(d) ensure the continued acceptability of the benefit-risk ratio referred to in Sections 1 and 9 of Annex I; and
(e) identify possible systematic misuse or off-label use of the device, with a view to verifying that the intended purpose is correct.
Although the MDR is clear about the pillars of PMCF, there is a lot of room within each for interpretation by the manufacturer. We believe that the PMCF can be set up to support your company, based on the classification of your devices.
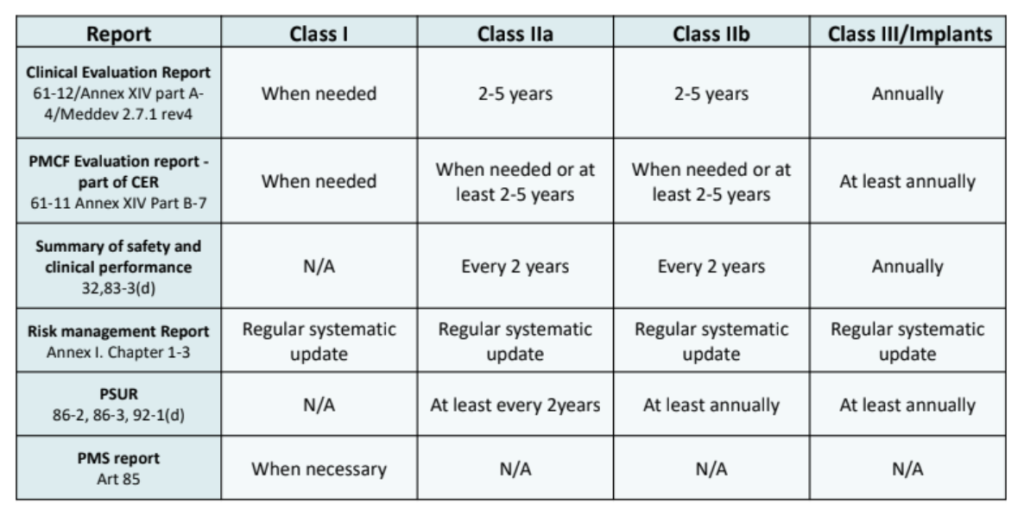
How can PMCF be used to your organization’s benefit?
Not every device has the same requirements. The scope of PMCF should be based on the device’s type and risk classification. It is important to note that the MDR does not necessarily require that clinical studies be performed to collect clinical data. In some cases, it is sufficient to draw on observations from commercial use. In other cases, a more controlled study setup will be required. PMCF studies might include:
- Literature reviews
- Focus groups
- User feedback during training
- Customer surveys (ePRO / eCOA)
- Device tracking/implant registries
- Clinical studies (including Investigator Initiated Projects)
Now, let’s dive into the three key benefits of PMCF and how it can create value for your company:
PMCF Benefit 1: Stay ahead of the competition
Manufacturers invest in device development under the assumption that devices will be marketable for a substantial period of time. When on the market, however, devices will face competition from newer devices reaching the market. It is therefore important for device manufacturers to stay in touch with the clinical use and performance of their device. PMCF helps you obtain data on how the device is used and its effectiveness in different scenarios. PMCF can also provide insight into possible off-label uses. This data can help you refine user information, update settings, make improvements, or find ideas for potential new indications.
PMCF Benefit 2: Obtain data for business use
Even though notified bodies do not always ask for a full clinical study for CE approval, your end-users might. Unfortunately, obtaining the CE mark does not guarantee anything. End-users might be skeptical about the product’s clinical or economic benefits. If skepticism exists, this can have serious consequences for device turnover and pricing. Collecting data on clinical performance and health economics can substantially improve the business case for your device. You can, for example, compare the performance of your device against that of a competitor. You can also present data at conferences or in scientific publications, and use it for negotiations with buyers.
PMCF Benefit 3: Opportunity to connect with your customer
Ultimately, the goal of medical devices is to improve patient health. PMCF can help you connect with both physicians and patients through feedback surveys. Companies could, for example, organize focus groups for intensive private feedback. Another option would be to create a social media community on Facebook, Twitter, or LinkedIn where patients can share their experiences.
More Data Really: How Castor EDC can help you succeed at PMCF
The core of PMCF lies in capturing a lot of clinical data. Using a centralized system that captures data from any source and device is of critical importance. Castor EDC helps you with just that. Companies and manufacturers can easily capture and store clinical data in compliance with all the required standards and regulations.
With Castor, you can send patient and physician surveys, track device progress, monitor all changes made to your study, and capture high-quality data. Having all clinical data, be it device, eCRF, ePRO, or eCOA, integrated into a single system makes it easily comparable and accessible for internal communication, device development, and audit purposes.
Castor’s technology helps you maximize value from your study data to efficiently get your devices to market and help them stay there. Castor can also help you set up your PMCF registries, set up patient questionnaires (Castor ePRO), and connect you to our network of experts.
Looking to set up your PMCF registries? Schedule a demo with us today.



