CEOs of medical device companies have an intuitive sense of what they need from their clinical data. However, when designing their clinical trials, they often focus on proximate goals such as receiving CE registration. It is only natural that they attempt to reach these goals at the lowest cost possible.
A company might, for example, design its study end-points around safety and efficacy, while customers might want to see data on clinical benefit. Also, pricing and reimbursement might ultimately need to be supported with clinical and health economic data. If a study is designed with this in mind, this might save costs and increase revenue. In addition, if you know your Post Market Clinical Follow-up (PMCF) requirements, you might design your clinical trial such that forms and study design can be recycled later on. To save costs, many companies still perform their study using a combination of paper and Excel. In our blog post Paper vs. eCRF we have shown that using paper is actually not cost-effective in the long run. Because of short-term study designs and inconsistent methods of collecting data (e.g. paper, Excel, other solutions), companies often lack a clear overview of their clinical data. This leads to companies not leveraging the full potential of their clinical studies.
We recommend having a plan in place for collecting clinical data for the entire lifecycle of your devices and investing in a long-term EDC solution to collect and store this data. In this paper we go over four reasons why this makes sense.
1. Draw conclusions from different datasets
Throughout the lifecycle of your device you will collect clinical data at different points in time. The first clinical data you collect is design verification, which might be as simple as patient interviews. Health economic data might look at time reduction in the clinic or benefit to patients. Post-market clinical follow up might be large scale registries with periodic patient-reported outcome surveys (ePRO/eCOA). In the end, all data is part of a single body of evidence that makes the case for the need for your device.
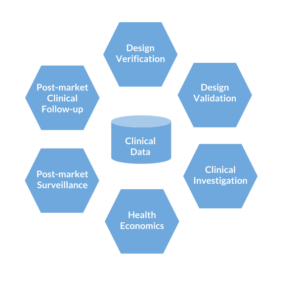
Figure: Clinical data collected during the medical device life cycle
Having all data on a single platform allows you to visualise and report these benefits to regulators, investors, and customers in a suitable format. It also allows your company to analyse relations between studies and compare trends. Because data is standardized, it will be reusable for future studies.

2. Increasingly stringent regulation make investment in an EDC system a must
Under the Medical Device Regulation (MDR), clinical data collected during both clinical investigation and post-market surveillance make an integral part of your device’s Clinical Evaluation. Clinical data collected over the life cycle of your device all ultimately feed into the Technical Documentation. The Clinical Evaluation Report needs to be updated periodically with data collected in the post-market phase. Having access to all data in one place makes this process much easier. You will be able to create reports that are accessible from different locations, and data will be visualized in the way that is requested by Notified Bodies.
Using a single platform for data management is also a must when complying with Good Clinical Practice (GCP) and General Data Protection Regulation (GDPR). Audit trails, user access rights, and data encryption are impossible to control using different mediums such as paper, Excel or non-EDC cloud services. In other words, an EDC is highly recommended for compliance reasons alone.
3. Do not compromise between accessibility and security
Locking away paper in a vault can be very safe for data protection, however, security will always go to the detriment of accessibility. In a business in which data is one of the value drivers, it must be easily accessible, but without compromising security.
Clinical data is used in design decisions, regulatory reports, communication with Competent Authorities / Notified Bodies, investor relations, due diligence, and in potential audits.
Therefore it makes sense to invest in an EDC system that allows for on-demand data access and reporting for different uses, while offering military-grade data security and automatic backups.
4. Lower cost of data collection and ownership
Using a single platform for collecting and storing your clinical data will ultimately lower the total cost of ownership. As we discussed at length regarding eCRFs in clinical trials, and how the cost of collecting data using EDC is more cost effective because it lowers the time needed to clean up data.
An EDC allows you to collect various kinds of data in a single location, from electronic Case Report Forms (eCRFs), electronic Patient Reported Outcomes (ePRO), electronic Clinical Outcome Assessment (eCOA), Serious Adverse Events (SAE), and user feedback.
Conclusion
Companies often lack a clear overview of their clinical data, which can lead to extra costs and a loss of knowledge.
We recommend for medical device companies to have a plan in place for collecting clinical data during the entire lifecycle of their device. In addition, we suggest investing in a long-term EDC solution to collect and store this data, in order to leverage the full potential of your clinical data. Doing this will allow your company to
1. Store data in a single location for comparison and reuse;
2. Be compliant with relevant clinical and data regulations;
3. Have data both accessible and secure;
4. Lower the cost of data collection and ownership.



