Most researchers around the world use tools like Microsoft Excel, Microsoft Access, Google Forms, or SPSS for data collection in healthcare. Although these data capture tools can be helpful, they also have several shortcomings and limitations that might affect the study’s productivity and data security.
Advantages of commonly used data capture tools
Excel, Access, Google Forms, and SPSS rank among the most used data capture tools in healthcare as most researchers have used them at one time or another in daily life. Researchers’ previous experience with these general- purpose tools makes their use in clinical research very convenient, as no new tool training is necessary. Helpfully, these tools are designed to be intuitive enough that first-time users can quickly get up to speed. Additionally the fact that these data capture tools are widely available and often provided by universities and parent institutions explains their popularity among researchers.
For example:
- Google Forms makes it especially easy to collect information directly from patients, since it can be sent out as a survey and enables collaboration on eCRF designs with other researchers.
- Both Excel and Access allow you to import or directly link to data stored in other applications and databases.
Shortcomings and limitations of these data capture tools
Even though the above data capture tools may provide some advantages for data capture in clinical research they also have severe disadvantages that can slow down the productivity of clinical trials and might pose data security concerns.
1. Not intended for medical data capture
Even though the spreadsheet application Excel, database management system Access, and the statistical analysis application SPSS offer data input validation features, they were not specifically created to capture data for clinical research, which makes them cumbersome to operate and complicated to set up.
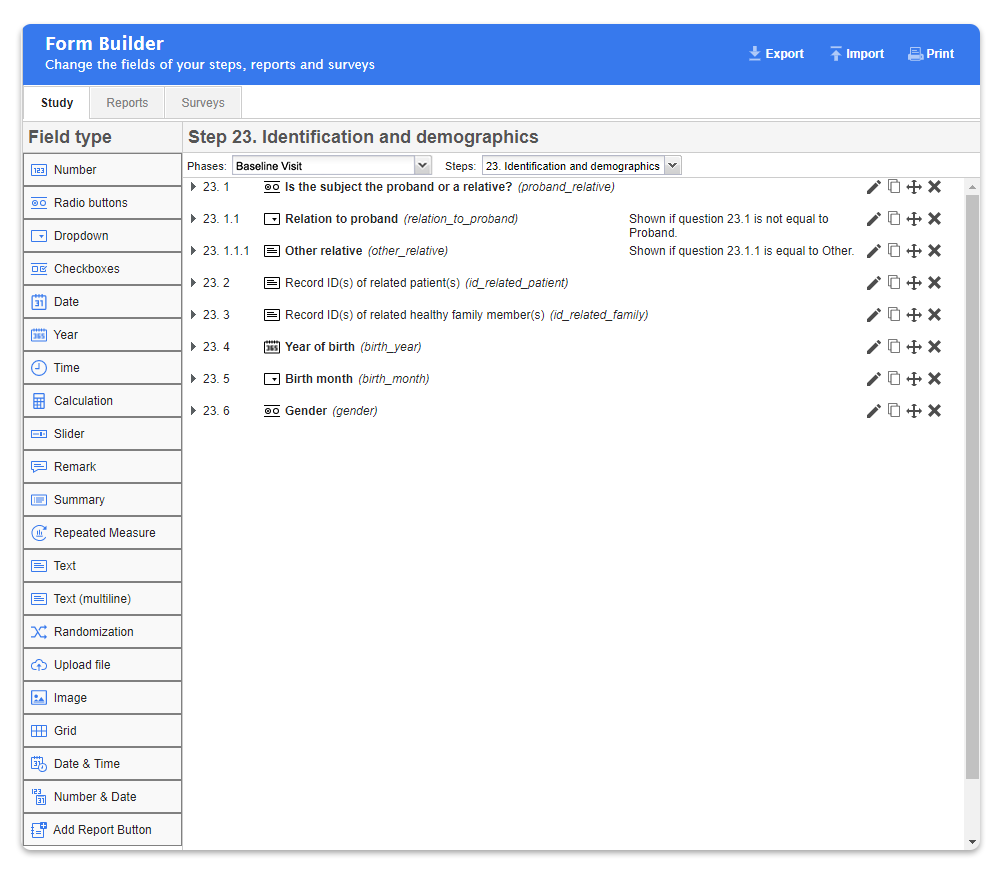
Advanced electronic data capture systems such as Castor EDC were specifically designed for medical research and solve many day to day problems researchers encounter while collecting data for their clinical research, such as:
- Easy drop down menus
- Ability to set up field validations and limits when designing forms to enhance your data input accuracy.
- Ability to set up field dependencies and perform advance calculations
- Ability to collect data in standardized format. This enables researchers and their colleagues to easily reuse data for replication and meta-analyses, combine datasets, and test new hypotheses (greatly reducing data waste and increasing the impact of research data!)
2. Not designed with researchers in mind
Excel, Access, Google Forms and SPSS do not offer special guidance for setting up scientific studies and research projects, and this is to be expected. But this can present a challenge for many scientific researchers.
At Castor, we have changed that. Castor clinical data collection tools are user-friendly because they are designed for researchers, by researchers. We provide you with easy step-by-step guidance on how to set up your study. With our cloud-based web applications, you can set up your study in just hours. Before you design your eCRFs or begin capturing data, you progress through a series of guided steps to set up your study and integrate your study protocols into a new study on Castor EDC.
3. No dedicated online resources for medical research
Dedicated guides and manuals on how to set up your studies in Excel, Access, Google Forms or SPSS are limited or non-existent. You might find an online forum that addresses your specific problem. However, finding information on advanced dependencies and validations will likely require hours of research.
Also, there is no guarantee you will find information that is specifically related to your study. You are basically on your own when it comes to solving complex problems associated with data validation, randomization and other issues that are important to scientific researchers.
In addition, these tools do not offer guidance on eCRF design. Excel, Access and Google Forms do not provide value added features, templates or productivity tips. As a result, many PhD researchers spend a lot of their precious time—which they could better spend on actual science—designing forms using various applications, formatting worksheets and other IT tasks.
You should not be wasting your time on these tasks.
In contrast, Castor offers a wealth of online resources to guide you every step of the way, including:
- A one-hour online workshop on all the Castor basics to empower you to easily create your own forms.
- Our Form Exchange allows you to exchange calculations and forms with other researchers using our platform. Simply download your SF-36 or EQ-5D and plug it into your study.
- The Castor Calculation Helper will help you get your advanced risk score calculations up and running in no time.
- Our online manual allows you to quickly find help on any topic.
4. No one to ask if you get stuck
Besides Google, who do you ask if you get stuck in Excel, Access, Google Forms or SPSS? Some universities offer support for SPSS, but it might take a long time before you receive an answer.
Our Customer Success Team will support you along the way.

On average, our help desk responds to your queries via [email protected] within one hour.
Feel free to describe your technical challenges to us and we will see how we can solve them. We guarantee an answer to your query within 24 hours on weekdays regardless of your time zone.
We take your problems and time seriously. We want every researcher to have access to a professional EDC system, not only those working in sponsored trials or pharmaceutical companies.
To us, helping medical researchers is just part of our job, not a burden. Since all of our customers are medical researchers, your problems and issues have likely been highlighted before.
5. Neither secure nor compliant
Because they were not developed with medical research in mind, these general purpose applications are not secure and not compliant with legislations. With regulations tightening across the world, compliance with, for example, ICH-E6 – Good Clinical Practice (GCP), is critical for all researchers involved in clinical trials.
For example, when you use Google Forms, you do not know where your data is being stored. This is a breach of national and European regulations for conducting medical research.
Castor is compliant with all applicable laws and regulations for your clinical trials: ICH-E6 – GCP, 21 CFR Part 11, HIPAA, EU Annex 11, and the European Data Protection Directive. We have been explicitly validated by several hospitals and research institutes. Contract Research Organizations (CROs) widely use our Electronic Data Capture (EDC) platform as well.
Castor also includes an audit trail that logs every action taken in the system, in accordance with GCP guidelines.
You can select in which of our data centers you want to store your information, and we will ensure it never leaves there without your permission!
Visit our GCP overview page and Security statement to find out how our audit trail, reason for change functionality, and secure data storage help keep your data safe and compliant.

6. No support for multi-center trials
Sharing Excel, Access and SPSS files between users is messy. More importantly, it can compromise data quality and accuracy. It can also easily lead to privacy violations and data security breaches.
Excel and Access allow for assigning authorization levels and locking of different parts of the worksheet or database. But these tend to be complicated. Also, lacking proper rights management means unlocking a worksheet or database can cause all data to become accessible to everyone.
Google Forms enables collaboration but is not equipped to handle rights management necessary to ensure data is only disclosed to authorized persons.
In contrast, Castor supports everything you need for a multi-center, complex trial. We have built-in features that make it suitable for multi-center Randomized Controlled Trials (RCTs) and other studies requiring collaboration and advanced rights management.
- Each record in Castor belongs to an institute/center. Authorizations can be set up with specific details so members of your study team can only access the data they are permitted to access. You can assign authority separately for each user to Add, View, Edit, Delete, Lock, Export, Query, Randomize or Sign eCRFs, or parts of them.
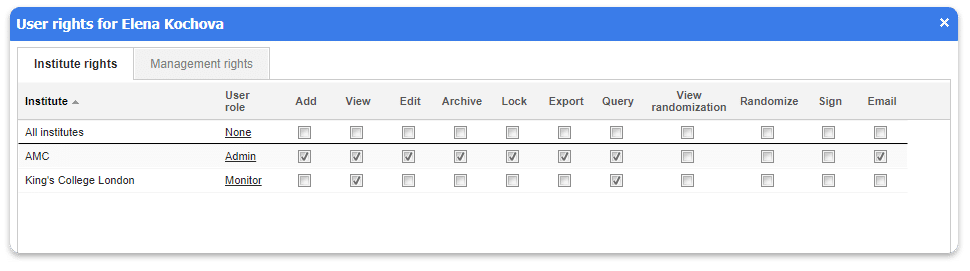
Each Record in Castor belongs to an Institute.
- You can also restrict management rights to manage records, forms, users and settings, as needed. If you are involved in many different studies, they will show up as a list when you log in to Castor.
- If you are involved in many different studies, they will show up as a list when you log in to Castor.
7. No support for randomization
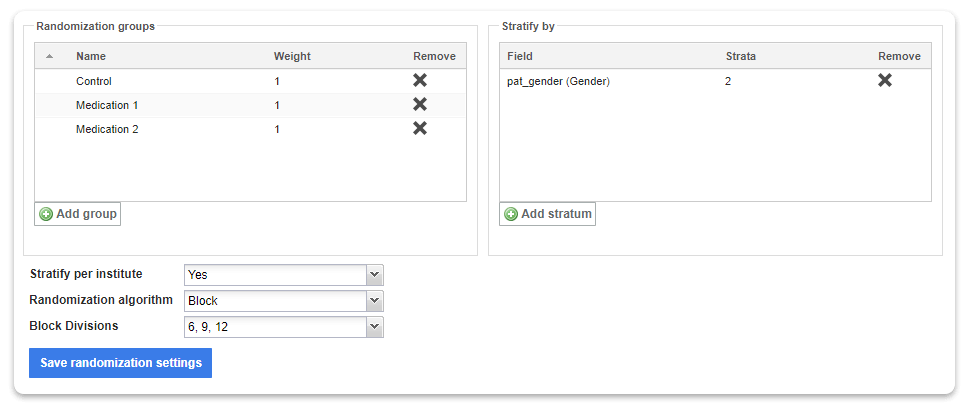
Setting up randomization in Castor is easy.
If you are running a Randomized Controlled Trial, you need to carefully organize how you randomize your patients to ensure your study is being conducted in accordance with your protocol. None of the alternative tools discussed in this article have build-in randomization. So you need to resort to an external tool and feed the results back into your database to effectively randomize.
In Castor, you can use a variable block randomization method that only authorized study team members can view. Read more about this in our introductory blog article on randomization in medical research.