An avalanche of data from so many real-world sources is available today, with the promise of more data generated daily—so why not use it?
Real-World Evidence is evolving before our eyes
Real-world evidence (RWE) is a hot topic with the FDA and those submitting to the FDA, and it is a topic that promises to grow in interest and relevance. Since the Cures Act of 2016, the FDA has been looking for and finding reasons to pay attention to RWE made up of Real-world data (RWD). With increasing urgency, RWE has been finding its way into regulatory submissions for new products.
RWD is rising to the authoritative level of randomized clinical trials (RCT)—the standard-bearer of definitive evidence. RCTs are designed and administered to produce the results needed to meet stringent clinical criteria, while RWD generally collects data to support something other than a clinical trial. RWD is if anything, an afterthought when it comes to supporting clinical trials. But with increasing amounts of data becoming more available every day, it was only a matter of time before the rigor and methodologies that power RCTs get designed into the RWD that is churning out day in and day out. The RWE generated by RWD will not always be only an afterthought.
In this article, we want to show the gaps between how data is collected in RWE and RCT and how RWE is quickly finding ways to catch up: from applying rigor to RWE by reusing data models and repurposing methodologies from RCTS to creating fit-for-purpose methodologies for post-market surveillance.
Adding rigor to RWE

The FDA and other regulatory bodies encourage using RWE to bolster the authoritative stance of the submissions they receive from companies. While RCTs may constitute the bulk of submissions, RWE is increasingly recognized by the FDA as part of the evidence package and can help round out the submission as “substantial evidence” or “primary evidence,” both of which make a case for product safety and efficacy. RWE can also provide “supportive evidence,” which reinforces the case of the submission.
While the FDA looks at RWE as a part of the evidence package, the agency does not allow RWE to meet a lower threshold of evidence compared to studies using RCTs. In fact, not all RWE was accepted as the evidence it was intended to provide. In a review of FDA documentation, Papura et al. observed the FDA citing the use of the three categories of evidence noted above (substantial, primary, supportive) to approve new license applications. They also noted that the RWE did not always rise to the level of supporting the FDA’s benefit-risk assessment framework.
To meet the exacting needs and frameworks of the FDA, it makes sense to look at adopting the science behind RCT. In particular, the methodologies that make RCTs must be adapted to produce fit-for-purpose RWD (that is, data that meets the more stringent criteria found in an RCT). For instance, RWD must meet the same relevance and reliability standards used for RCTs.
- Relevance: The data should represent the intended population. The data should capture exposure, and confounders should be captured and measured.
- Reliability: Ensure accuracy, completeness, and consistency
Identifying and adjusting for demographics, socioeconomic and insurance status, disease severity, comorbidities, and other confounding factors are also important. One of the important questions facing the use of RWE is the question of systematic bias.
Evaluation for bias includes questions like:
- Are the patients in the dataset representative of the population of interest?
- Are critical data fields representing exposures, covariates, and outcomes present? If not, are these variables able to be algorithmically derived using data fields that are present?
- If more than one data source is required, are data fields present that permit accurate linking at the patient level?
- Are there sufficient persons and follow-up time in the data source to demonstrate the expected treatment effect, including adequate capture of potential safety events?
A real-world dataset is relevant if it is robust and representative of the population of interest
– Center for Drug Evaluation and Research
RWE plays a bigger role in post-market clinical follow-up (PMCF)

Post market clinical follow-up (PMCF) studies have always played a large role in ongoing safety and efficacy monitoring. Post-market surveillance helps demonstrate the real-world safety and effectiveness of a drug or medical device. After all, it would be impossible to know all the potential side effects of a drug based only on the preapproval studies.
The FDA Adverse Event Reporting System (FAERS) is one avenue for monitoring the PMCF experience with a drug. MedWatch is another program that allows for the voluntary reporting of serious reactions and problems with medical products. Clinical data registries are another important set of tools for tracking the details of disease or pathology progress. In addition to these and other programs, RWE is playing an increasingly important role.
Real-world evidence is traditionally used for PMCF studies. The use of RWE in postmarket surveillance adds richness to the data collected from clinical trials. Swift et al., in their look at the innovative uses of RWE, noted potential deficiencies of RCT. In particular, some inferences that arise from RCT can limit the ability to individualize for specific clinical scenarios in real-world medical practices. So planning to include RWE helps expand the application of evidence-based medicine.
The use of RWE for post-market surveillance is already a natural fit and becomes even more relevant as RWE is fit-for-purpose for the many stages of a therapy’s life cycle.
Tokenization and RWE
Tokenization takes RWE even further by making data available on a broader scale. Tokenization uses a unique, anonymized identifier (a “token”) to track a patient’s information without linking that information to the patient’s identity. Tokenization allows another researcher to link external data sources to the original study with a different (or adjacent) set of questions that examine the data from a different vantage point. For instance, according to Deborah Borfitz, writing in Clinical Research News, a study that used “time to next treatment” as an endpoint could be reviewed again by examining claims data, which could identify the time the participant was on the intervention. Tokenization allows creative thinking around the kinds of questions that can be asked of data.
Tokenization of RWE will prove useful in broadening the data sets available in the post-market surveillance phase, creating new avenues for verifying efficacy. We can expect faster and more efficient analysis as the tokenization of RWE draws from a variety of data sources, including EHR, claims data, and even social media posts. Results may include faster responses to safety concerns and better risk management strategies.
How Castor can help your real-world data capture strategies

RWE deserves to move out of the domain of afterthought. It rightly deserves a front and center seat in the study pipeline, which is precisely what the FDA and other regulatory bodies are eagerly pushing for. Beyond post-market surveillance, FDA considers RWE studies as part of the evidence package for submissions seeking authorization to market new medical products, including new drug applications (NDAs) and biologics license applications (BLAs).
Castor can help in the creation, collection, and analysis of RWE. Our experience with mobile apps, eConsent, and wearables can increase recruitment rates (by 50-65%) and patient retention (40% higher), which results in more and better RWE over time.
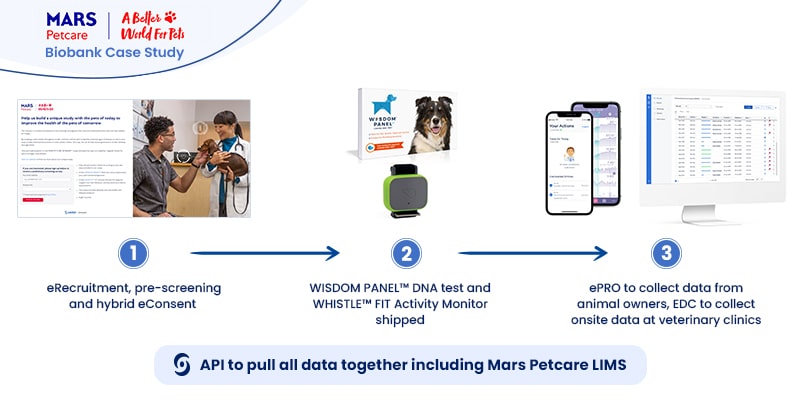
Real-life example: MARS Petcare Biobank
The MARS Petcare Biobank is an ambitious large-scale, longitudinal study to collect health and disease state information from 20,000 pets (10,000 cats/10,000 dogs) over ten years. The real-world, open-access data will be available for researchers, with raw genome sequences to be released as they become available. The study team was well aware of the enormous amount of information they would handle by collecting data from 20,000 wearables.Mars Petcare turned to Castor to help collect and process the tsunami of real-world data from tracking 20,000 pets in more than 50 sites for ten years. Real-world data would be gathered from WISDOM PANEL™ DNA tests to determine the pet’s breed background and the WHISTLE FIT™ activity monitor—both shipped to the pet owner’s home.Castor electronic data capture (EDC)—supported by the use of the RESTful API—helped to quickly capture and collect trial data produced by the many wearables and seamlessly integrate that data into the study. Castor products like eConsent and ePro helped speed initial phases of the study:
- eConsent, by creating hybrid eRecruitment methods (online landing page & pre-screening surveys delivered online or in-person on paper).
- ePro greatly simplified the participant-reported outcomes, which
- EDC would collect onsite and in a single onsite repository
To learn more, watch Castor’s latest webinar “The role of patient-centric technologies & tokenization in RWE studies” as our industry experts from United Biosource (UBC) and Datavant discuss how a modular approach to clinical technology can help you conduct an RWE study.


