Though technologies that support decentralized clinical trials (DCT) have been readily available for years, meaningful industry adoption has lagged. In the past, researchers have generally avoided DCT-enabling technology, such as eConsent, and have been slow to upgrade to more advanced options even when they use electronic trial management systems.
While COVID-19 has boosted the urgency of adopting decentralized or partially-decentralized (“hybrid”) research approaches, the perceived roadblocks to implementation remain, including a fear of non-interoperability between technology solutions and the challenge of initiating change (“where do I even start?”).
Castor recently sat with Craig Lipset, a recognized leader of innovation in clinical research to understand his perspective on overcoming DCT and hybrid trial roadblocks. During his time as Head of Clinical Innovation at Pfizer, Craig was involved in multiple industry firsts, from the first fully remote/virtual clinical trial for a new medicine to the first returning of results and data to research participants.
Though technologies that support decentralized clinical trials (DCT) have been readily available for years, meaningful industry adoption has lagged. In the past, researchers have generally avoided DCT-enabling technology, such as eConsent, and have been slow to upgrade to more advanced options even when they use electronic trial management systems.
While COVID-19 has boosted the urgency of adopting decentralized or partially-decentralized (“hybrid”) research approaches, the perceived roadblocks to implementation remain, including a fear of non-interoperability between technology solutions and the challenge of initiating change (“where do I even start?”).
Castor recently sat with Craig Lipset, a recognized leader of innovation in clinical research to understand his perspective on overcoming DCT and hybrid trial roadblocks. During his time as Head of Clinical Innovation at Pfizer, Craig was involved in multiple industry firsts, from the first fully remote/virtual clinical trial for a new medicine to the first returning of results and data to research participants.
A three-tiered approach
While a multitude of technologies can be involved in DCT, the common core components are typically eConsent and ePRO, technologies supporting direct engagement with the participant through web and mobile. While the market offers many sophisticated tools and solutions for the management of clinical trials, these are the cornerstones for decentralization. It bears repeating that the solutions chosen for these and other needs must be compliant with applicable regulations. It can sometimes be confusing to assess the compliance of technologies for which diverse global regulations have not quite caught up, so these questions should be asked early on in the process. The recent COVID-19 research initiatives have moved DCT adoption into the public eye, as they offer powerful and viable support for research continuity. Still, the associated choices are urgent and complex; trial components such as recruitment and enrollment present challenges even in non-pandemic times. What is required to rapidly implement the most basic decentralized or hybrid trial? And from there, what does the space require to become not only competitive but an industry leader? To start, Lipset suggests considering DCT technology as three separate tiers: a base level to get going; a state-of-the-art integrated space; and finally, a full ecosystem equipped for groundbreaking research. You can start a decentralized trial with two technological building blocks to support participant consent and report their trial data:
You can start a decentralized trial with two technological building blocks to support participant consent and report their trial data:
- Electronic consent, (eConsent): eConsent is the electronic form of the traditional clinical trial consent process and can be obtained remotely from participants’ homes.
- Electronic patient-reported outcomes (ePRO): ePRO allows patients to answer questions and report on their health through an electronic device, such as a smartphone or tablet at any location or time, thereby automatically decentralizing self-reported data.
- Participant portal for patients to get information (such as upcoming visit expectations), sync with study staff, and collect more types of data
- Video conferencing to support virtual visits with study staff and keep communication open and interactive
- Integration between your software solutions to streamline data flow, enable real-time triggers and alerts, and improve data monitoring
- Support for home health visits such as visiting nurses to assist with triggering, scheduling, and data capture
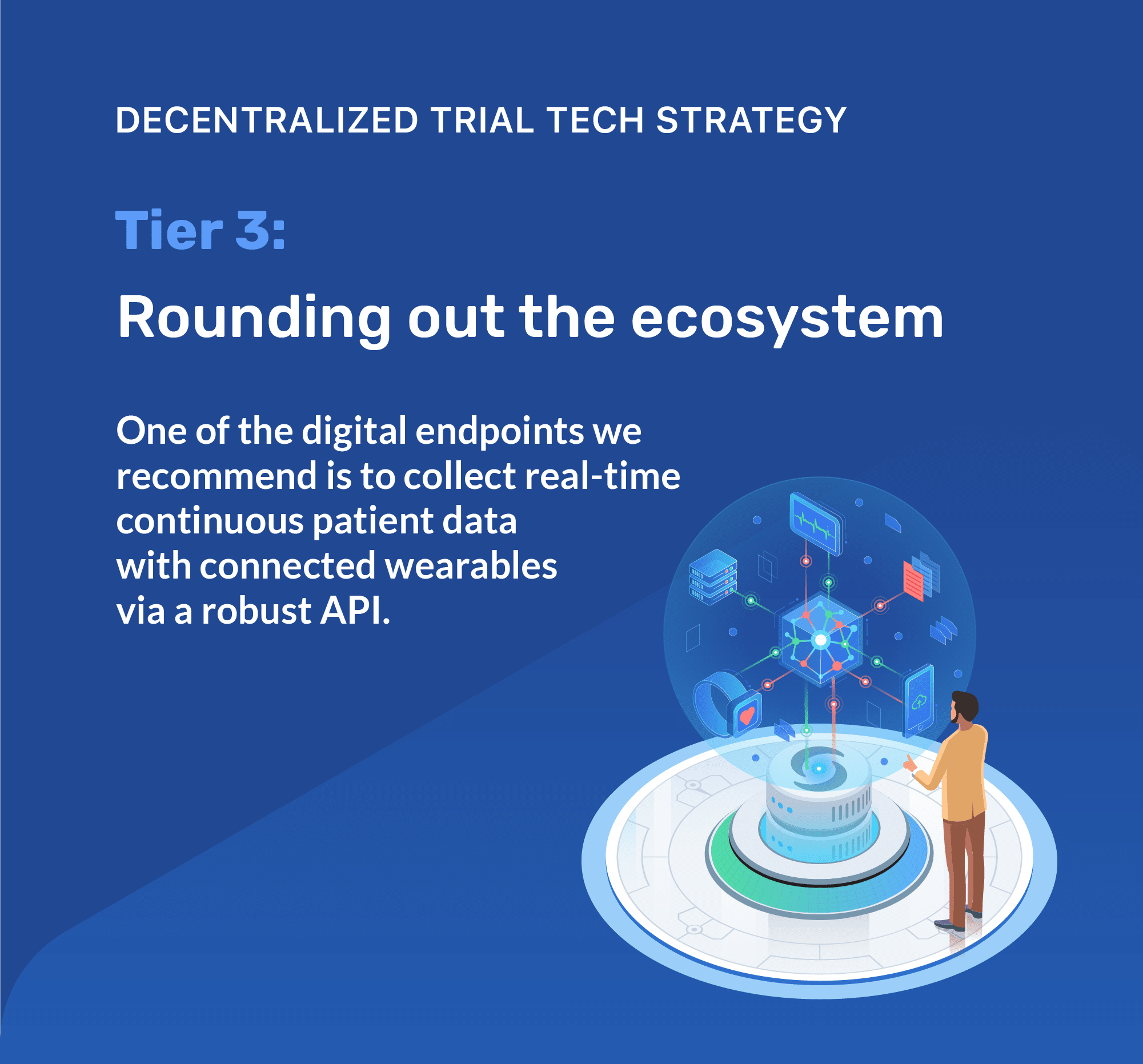
- Collect real-time continuous patient data with connected wearables via a robust API.
- Capture medical photographs
- Accommodate DICOM images
Three more pro tips
Let’s review some additional DCT and hybrid trial best practices from Craig, which will also help your DCT journey.- Focus on participant choice and comfort. Allow interested participants, where possible, to go to a physical site if they choose, or remain remote at their convenience. Seek ways to include the participants’ investigators in their interactions, such as video touchpoints to provide participants both connection and assurance throughout the trial.
- Do be open to multiple communication options. A phone is the primary communication device needed for DCT. Research organizations should ask, “If we start with the phone, can we do video? If we do video, how do we incorporate chatbots or virtual consultations? If we do self-reported data, how can we capture more wearable data? If we do that, how can we use voice to capture more experiential data from patients comfortably?” Don’t be afraid to embrace a wide range of communication tools. Remember, a patient-centric trial is about offering the participant choice.
- Don’t get too comfortable with the status quo. Your industry peers will also be implementing new methods, so consider how your organization might innovate in your particular research niche, and lead in creative and groundbreaking ways.