Categories
Connect with us on LinkedIn
The real-time monitoring dilemma
Offline eCOA capability creates regulatory tension with EMA device-as-source requirements while eliminating real-time monitoring capabilities that drive 80-95%...

Is Your eCOA UAT Stuck in Time?
Modern clinical trials increasingly rely on complex, time-sensitive logic within eCOA systems, such as narrow compliance windows and...

The End of the “PRO Tax”: Top 10 Commercial PROs & their cost-effective alternatives
The clinical trial industry faces a "PRO Tax"—the high costs and operational delays associated with licensing and implementing...
The Silent Saboteurs: Why Rater Drift and Site Unpreparedness Cost CNS Trials More
CNS trials fail not from technology limitations but organizational factors. Research reveals 55% of sites lack adequate eCOA...
Hospital-Based eCOA Implementation: Real Challenges in Infectious Disease Trials
Hospital-based infectious disease trials face unique eCOA implementation challenges including IT security delays, staff turnover, and patient acuity...

Implications of Assessing Overall Survival in Oncology Studies
The FDA’s August 2025 draft guidance reshapes oncology clinical trials by requiring pre-specified overall survival (OS) analysis in...

The Patient Experience Paradox: eCOA Strategy Overhaul
Discover how the EMA’s new Patient Experience Data (PED) guidance and the EU HTA Regulation are reshaping evidence...

On-site ePRO in Action: A Recap of Castor’s Product Spotlight
Castor’s on-site ePRO adds in-clinic PRO capture to the ePRO/CDMS workflow you already use. Staff use a site...

On-site ePRO
Castor’s On-site ePRO ensures higher flexibility for both study designers and participants, by maximizing opportunities for data capture.

Castor On-site ePRO
Download our On-site ePRO factsheet to view an overview of our services.

EQ-5D in European Trials: When Generic QoL Measures Actually Matter
Analysis of 735 FDA drug approvals reveals 0% EQ-5D labeling inclusion, while European HTA bodies demonstrate 18% technology...

Castor Product Spotlight: New On-site ePRO
Watch this on-demand Product Spotlight to see Castor’s On-site ePRO in action. Our experts show how on-site capture...

Navigating the eCOA Vendor Landscape in 2025: What Clinical Teams Really Need to Know
The eCOA market reached $2.27 billion in 2025 with 16.1% projected growth, yet vendor selection remains critical for...

Optimizing eCOA in Oncology Trials: Patient-Focused Data Capture for Cancer Research
Oncology leads clinical innovation with 7,747 trials registered through 2022, yet only 7% of cancer patients participate. This...
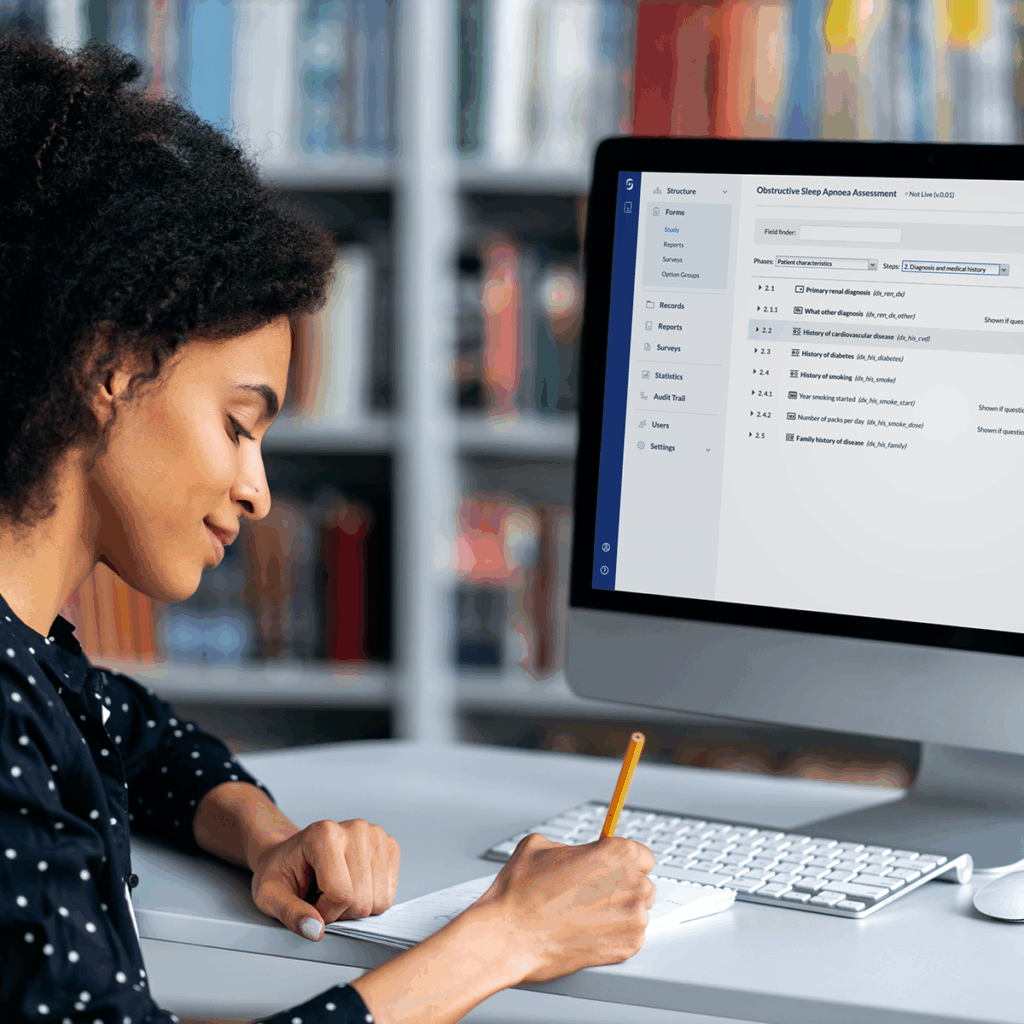
ePRO, eCOA 101: Everything You Need to Know About ePRO and eCOA
Electronic Clinical Outcome Assessment (ECOA) and electronic Patient Reported Outcomes (ePRO) represent far more than digital surveys in...

Why eCOA Still Fails in Clinical Trials: Practical Strategies to Fix Baseline Data Problems
Electronic clinical outcome assessments (eCOA) were supposed to solve data quality and amplify the patient voice. Yet missing...
How a 3-Person Team Got to Phase 3 — Without the Overhead
Gameto proves you don’t need a large CRO or a floor of data managers to run complex global...

Broken at Baseline: Why eCOA Fails in the Real World (and Why We Don’t Talk About It)
Why are baseline PROs still going uncaptured in 2025? Join Ari Gnanasakthy, Katja Rudell & Derk Arts to...