
Categories
Connect with us on LinkedIn

Castor Professional Services: Expert Clinical Study Builds
Castor’s Professional Services team offers end-to-end support for clinical trial implementation, including EDC build, eCOA, and data management....
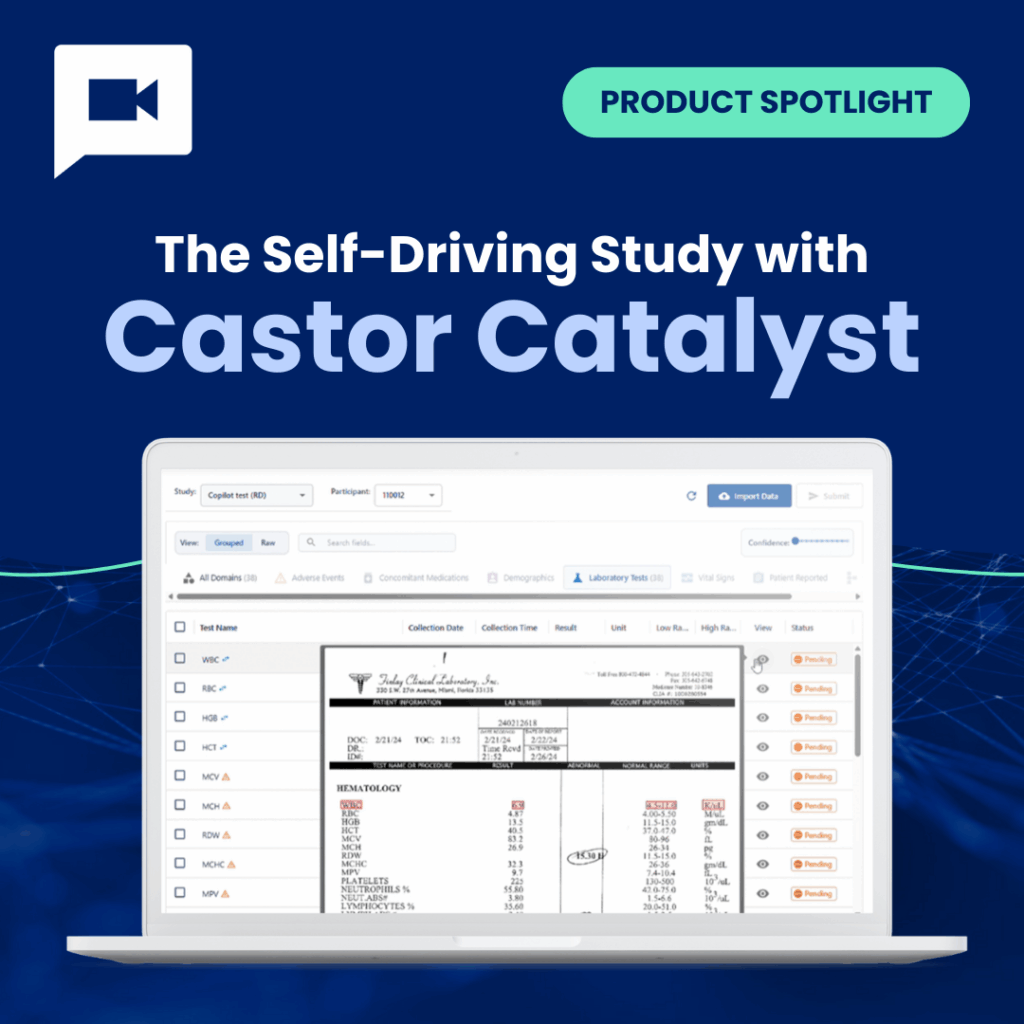
Product Spotlight The Self-Driving Study with Castor Catalyst – On Demand
Join Castor CEO Derk Arts to explore Castor Catalyst, the agentic AI platform built with Google Cloud AI....

From Months to Hours: MMC’s 5-Hour EDC Builds
Discover how a specialized CRO delivers first-in-human device studies at speed with Castor

Castor Catalyst
See how Castor Catalyst automates the transformation of real-world data into submission-ready study datasets. In this on-demand demo,...

How a 3-Person Team Got to Phase 3 — Without the Overhead
Gameto proves you don’t need a large CRO or a floor of data managers to run complex global...

From Hype to Health: What Sword Health Got Right About Evidence
Sword Health didn’t follow the typical digital health playbook. Instead of rushing to market, they invested early in...

Oncobiomix: Slashing Costs and Elevating Data Quality
Discover how Oncobiomix used an integrated platform for eConsent, EDC, and ePRO to save $100K and streamline data...

Product Spotlight: What’s New in 2025?
Discover how Castor's latest product updates in 2025 can improve your day-to-day operations, and the participant experience.

Castor Supports LLS in Study to Improve Blood Testing
Castor and LLS launch a study using AI and remote blood sampling to improve care for patients with...
Building a Safer, Smarter Future: A Tiered Approach to AI in Life Sciences
Adopt AI in clinical research with confidence using a risk-based framework that ensures privacy, security, and regulatory alignment.

Why a Life Sciences Museum Matters—And What It Can Teach Us About the Future of Medicine
What a Museum of Medicine can teach us about trials, trust, and how the public sees (or doesn’t...

Medical Coding in CDMS
With flexible configurations and exportable coded data, Castor makes medical coding fast, accurate, and efficient for any clinical...

Product Suite – Patient View
Complete consent forms, answer surveys, and communicate with her study team without needing to visit a clinic.

Flexible Remote DCT – Site View
Castor offers flexible and tailored solutions for clinical trials, from patient recruitment landing pages to automated screening and...

Flexible Remote DCT – Patient View
Flexible Remote DCT allows patients to participate in decentralized clinical trials (DCTs) from anywhere, offering convenience and reducing...

Castor CDMS 2025.1 New Release
Castor's Q1 2025 release boosts clinical trial management with improved reports, a modern UI, and enhanced Form Builder...
Build vs. Buy vs. Partner in Life Sciences: Making the Right Call
How do you decide between building, buying, or partnering? Learn key decision factors and red flags to avoid...

Do CROs Need to Reinvent Themselves in 2025? A Deep Dive with Greg Licholai and Derk Arts
High R&D costs aren’t due to a lack of innovation but inefficiencies in trials. Here’s how we can...