COVID-19 changed the conversation around enrollment and recruitment for clinical trials. With physical site visits severely impacted or impossible, sponsors and Contract Research Organizations (CROs) needed immediate solutions to continue research, like remote consent of participants. Enter electronic informed consent (eConsent)—not a new solution, but one that has taken center stage during this crisis and has now established itself as a core trial technology for the pandemic and beyond.
Castor’s CEO, Derk Arts, MD, Ph.D., recently led a virtual discussion for leaders in the research community. The following recap covers eConsent basics, how Castor’s new eConsent solution solves industry pain points, and how to best implement it.
Why is eConsent needed so much now?
Back in March, when sponsors and CROs were faced with COVID-19 and paused or stopped their clinical trials, they clearly could no longer run trials under normal conditions. The pandemic required the industry to rapidly identify solutions to resume research.
As the world braces for additional waves of COVID-19, that pressure continues and presents particular challenges for existing and new trial participants. Current trial participants, already burdened, may be even more negatively impacted by stopping trials. And enrolling new participants is even more difficult when most researchers cannot conduct face-to-face appointments.
eConsent, which enables the remote enrollment of participants, provides the solution.

What can we do as an industry?
The industry has considered digitizing clinical trials for years but has made slow progress. Almost a decade has passed since the first decentralized clinical trial employed remote trial technology. In this case, the Pfizer REMOTE study used entirely home-based participants and directly delivered the study drug to participants’ homes. Researchers recorded adverse event reports and efficacy outcomes using mobile devices and web-based measurement tools.
Although electronic patient-reported outcomes (ePROs) and eConsent have been available for some time, the majority of studies still don’t use them. With COVID-19 forcing research to be conducted remotely, the adoption of these technologies has increased dramatically. eConsent and ePRO, as well as an overall decentralized clinical trial technology strategy, keep your study’s participant enrollment and retention on track.
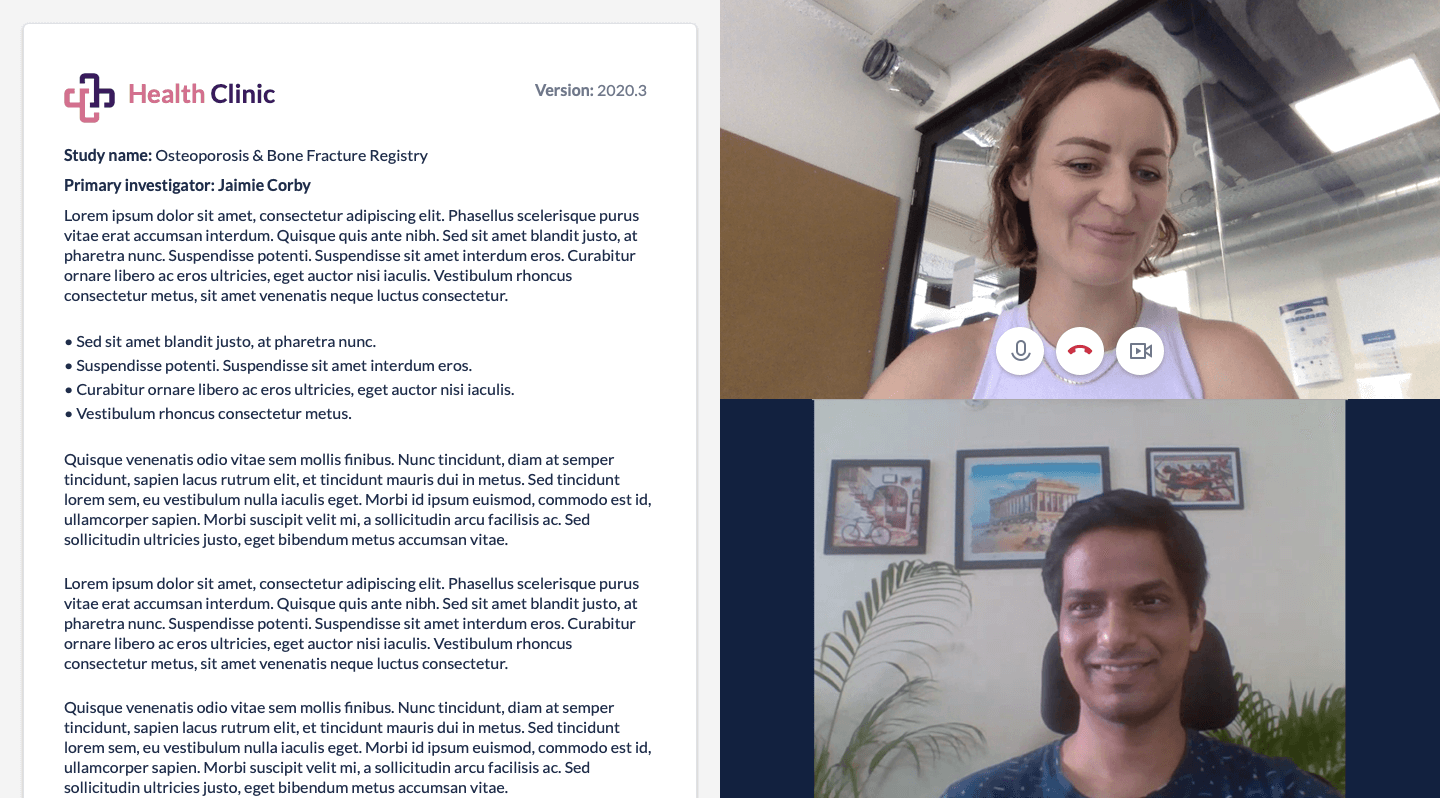
Remote consent challenges
When it comes to informed consent, sending participants information via electronic means is not new or complicated. Gathering participants’ signatures, however, can be challenging, as eSignature requirements vary by country. You must fully understand eSignature requirements for the countries where you run studies as you prepare for institutional review board (IRB) approval, regardless of whether your study requires full or expedited review. Studies eligible for expedited review may have less stringent consent requirements but typically still undergo an IRB, if more quickly. Learn more about the top eConsent questions here.
To get started, access Castor’s guide to eConsent readiness in the US, Europe, and Israel to learn more about the differing requirements and how you can prepare. As a global industry, we currently are not fully aligned in this area. The aim is that, over time, all countries will reach universal acceptance of eSignatures.
Checklist for eConsent implementation
The Castor team has significant experience working to implement eConsent for clinical trials and shares the following checklist to help you be implementation-ready:
- Confirm that the protocol discusses electronic consent and the IRB approves the use of eConsent.
- Validate that the consent forms are easy to navigate and contain hyperlinks to external resources such as video instructions.
- Understand how to manage eSignatures. Remember, eConsent must always include an eSignature unless eSignature is not accepted in the participant’s country. In that case, the participant needs to sign and mail an ink signature as well. You can find many white papers to guide you through this process and can read the FDA’s guidance here.
- Implement a solution to verify identity. You can employ several options, including uploading a document or requesting the participant to show a driver’s license or other ID while on a video call.
- Provide a process for each participant to receive a valid copy of the signed consent form.
- Ensure your electronic regulatory (eRegulatory) binders are organized and updated so that eConsent documentation is readily accessible for regulatory entities.

Components of an industry-leading eConsent solution
To learn more about what to look for in a quality eConsent solution, download our white paper “Guide to Using Electronic Data Capture (EDC) for Medical Device & Diagnostics Trials.” The white paper details how implementing a modern solution for electronic data capture can provide the winning edge for medical device and diagnostic trials, and provides the questions you should ask when identifying an EDC vendor.
What next?
eConsent fills a critical need for the industry, facilitating patient enrollment despite challenging circumstances. Ready to learn more? Schedule a demo and get started on your eConsent journey today.