
Categories
Connect with us on LinkedIn

ProlaC Study: 2019-20 Castor Research Award Nominee #6
When treating microprolactinomas (hormone-secreting tumors on the pituitary gland) is surgical treatment (endoscopic adenoma resection) superior and cost-effective...
6 Steps for Using eCOA / ePRO & Physician Surveys to Fulfill MDR PMCF
To ensure ongoing regulatory compliance under the EU MDR, medical device manufacturers must demonstrate the safety and performance...

Coach2Move Study: 2019-20 Castor Research Award Nominee #5
Continuing the nominations for the 2020 Castor Research Award, here is nominee #5. The Coach2Move study looks at...

EASED Study: 2019-20 Castor Research Award Nominee #4
The EASED trial studies sleep efficiency and how it is affected by aerobic exercise in patients diagnosed with...

Ivory Dentin Graft Material Study: 2019-2020 Castor Research Award Nominee #3
Continuing the nominations for the 2019-20 Castor Research Award, here is nominee #3. The Ivory Dentin Graft material...
Castor provides free access to data capture platform to support COVID-19 research projects
Amsterdam, Netherlands and Hoboken, New Jersey: February 17, 2020: Castor, a health-tech company that enables medical researchers to...
Introducing The New Castor Academy: Enroll Now!
We are very excited to announce the launch of the Castor Academy – our new eLearning platform. We...

Castor Appoints Dr. Ben Cons as Chairman of the Supervisory Board
Expansion of Supervisory Board Supports Global Growth Strategy Amsterdam, Netherlands and Hoboken, New Jersey: January 8, 2020:...
RSP Systems
Diabetes patients are recommended to test their blood sugar levels 4 to 10 times per day depending on...

APPROACH Study: 2019-20 Castor Research Award nominee #2
Continuing the nominations for the 2019-20 Castor Research Award, here’s nominee #2. The APPROACH study is a multicenter,...

Castor Establishes Advisory Board of Industry Leaders to Maximize the Impact of its Clinical Data Platform
Industry veterans including Craig Lipset will support Castor’s vision to advance the future of clinical research Amsterdam,...

Understanding GDPR and its impact on clinical research
Understanding the GDPR requirements and their impact on clinical research can be quite challenging. Watch an interactive webinar...

Castor Announces International Expansion to Meet Growing Demand for an AI-Powered Clinical Data Platform
Castor establishes new US operations, building on leadership position across the EU Amsterdam, The Netherlands and Hoboken,...

3 Key Benefits of Post-Market Clinical Follow-Up (PMCF)
Under the new Medical Device Regulation (MDR), companies planning to certify (or re-certify) their medical devices will face...
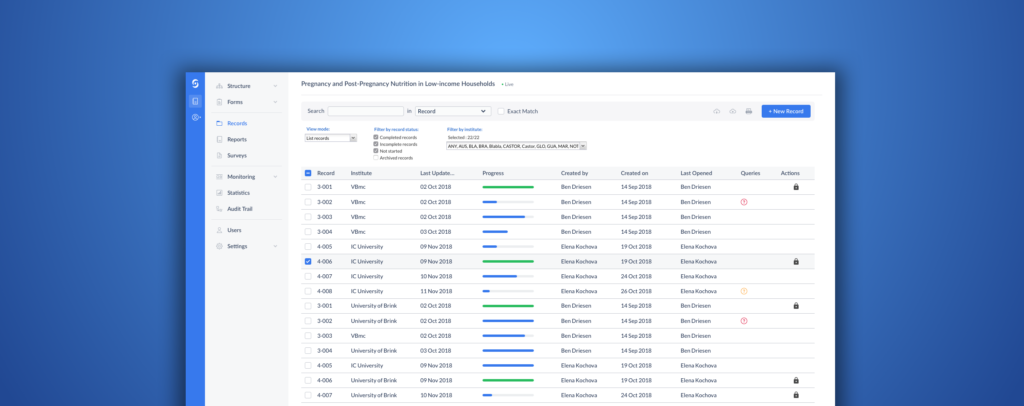
Introducing the new and improved Castor EDC
An electronic data capture (EDC) system that is easy to use and increases your team’s efficiency is instrumental...

Press Release: Castor Is Growing Throughout the Netherlands
A majority of Dutch academic medical centers are collaborating with Castor EDC to capture standardized medical research data...

BeBoP Trial: 2019-20 Castor Research Award nominee #1
Our customers are constantly striving to make the world a healthier place with their innovative research. At last...

ZEISS
ianTECH Inc. is a medical device company based in the United States that develops surgical devices, methods and...