Categories
Connect with us on LinkedIn

UKCA Mark: 7 Steps to Prepare Medical Devices for Brexit
In the wake of Brexit, medical device companies secure a UKCA mark as proof of conformity to place...
Decentralized Trial Tech Strategy In Three Steps
Though technologies that support decentralized clinical trials (DCT) have been readily available for years, meaningful industry adoption has...
Electronic Patient Reported Outcome (ePRO) Measures: Questionnaires & More
Patient reported outcome measures in clinical trials have traditionally been done on paper. Surveys are a common way...

User Acceptance Testing in Clinical Trials: Essential for Data Integrity
When it comes to building an instance on a clinical trials technology platform, User Acceptance Testing (UAT) is...
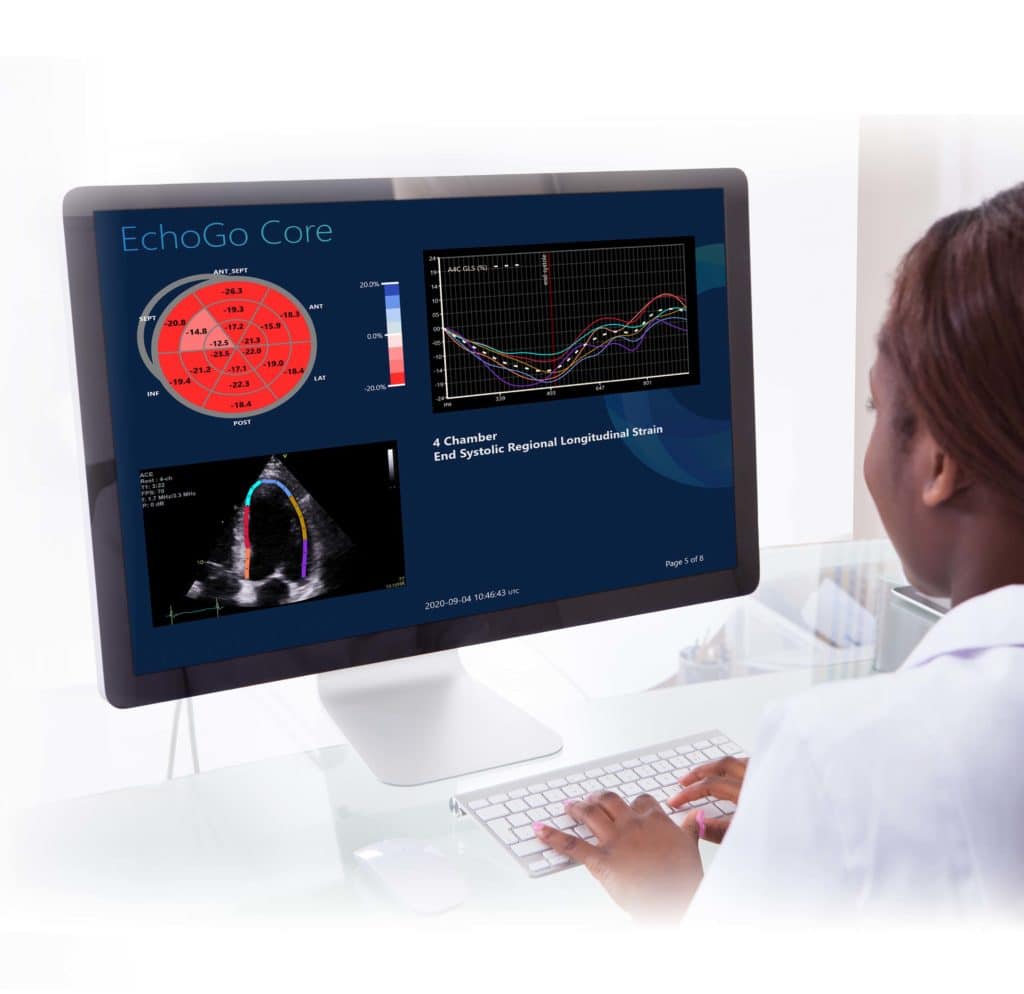
Ultromics: Powered by AI and Castor EDC
Ultromics, a diagnostics software company that develops cardiovascular imaging solutions powered by artificial intelligence, uses Castor for its...
How Data Collection Tools in Clinical Research Have Evolved
Most researchers around the world use tools like Microsoft Excel, Microsoft Access, Google Forms, or SPSS for data...
Guide to using Electronic Data Capture (EDC) for Medical Device & Diagnostics Trials
Download this guide to learn how to incorporating an Electronic Data Capture (EDC) system in your medical or...
Research Continuity During COVID-19
Watch this webinar for tips on how to ensure research continuity during COVID-19 by running your trial with...

Randomization & Blinding in Clinical Research Trials
Randomization is a key feature of clinical trials aiming for a valuable study outcome. In this article we...

Common shortcomings of clinical research data for PMCF
The following is an excerpt from our free whitepaper ‘Satisfying PMCF requirements by utilizing IIS data’. Click here...
Castor is now live in Australia!
To support our growing customer base in the APAC region, we established new hosting in Australia. By allowing...

eConsent – The Enrollment Solution for Decentralized Trials
Join this webinar to understand how eConsent works, where it falls in the regulatory guidelines & see a...

Castor Announces Partnership With Click Therapeutics to Support Decentralized Clinical Trials
Hoboken, New Jersey: September 2, 2020: Castor, a leading provider of clinical trial technology that automates the research...

How to streamline data capture and increase data quality using calculation fields in Castor
In this webinar, we explain how to do the following: Use variables in your study to calculate scores...

How to securely store sensitive medical data in Castor using encryption
In this webinar, we answer the following questions: Am I allowed to store personally identifiable information in my...

Castor Raises a $12M Series A to Further Their Support for COVID-19 Research
With 4,000 live studies and 2M enrolled patients across 90 countries, Castor will use the funding to further...

The Impact of ISO 14155 on PMCF Investigations Under the MDR
Download this white paper to prepare for the new MDR requirements requiring that PMCF investigations be conducted in...
An Expert Panel on Decentralized and Hybrid Trials, Pre and Post COVID-19
The world may have changed seemingly overnight, but clinical research and medical innovation must continue now more than...