
Categories
Connect with us on LinkedIn

What Will Trial Enrollment & Engagement Look Like in a Socially-Distanced World?
When the COVID-19 pandemic began, the industry needed to focus immediately on research continuity and determine how to...

Jumpstart Your Medical Device Preclinical Phase
The path to market for medical devices can be fraught with obstacles. One of the first challenges confronted...
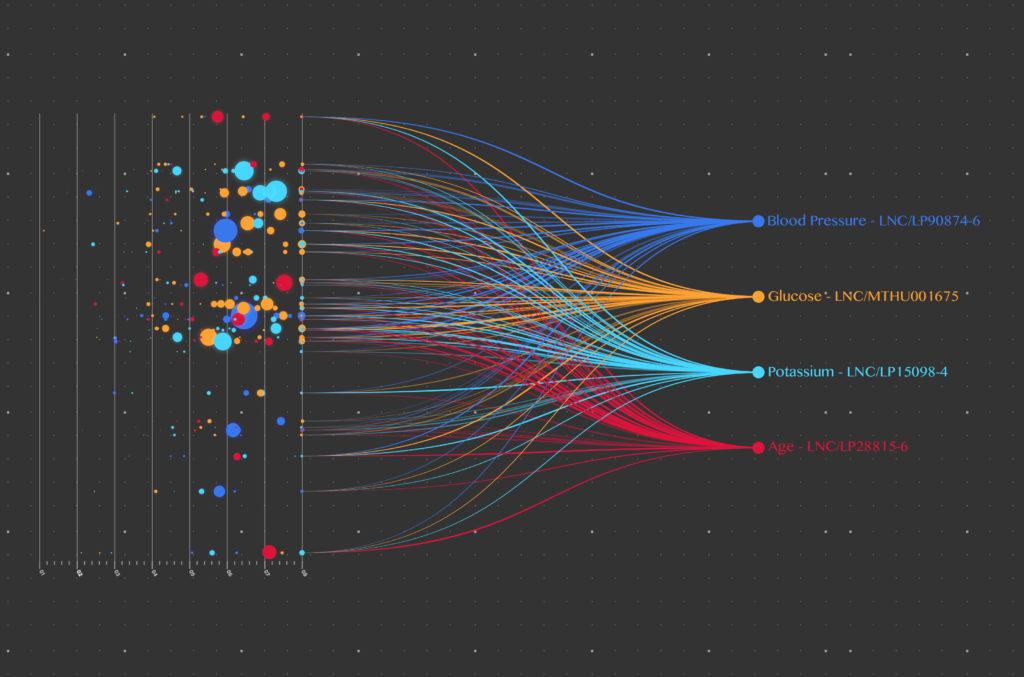
The future of data standardization in medical research: FHIR and FAIR
Medical research data holds the key to solving the world’s greatest healthcare challenges. It is therefore, one of...

6 essential steps to ensure MDR compliance before May 2021
A recent report surveying representatives from the medical device industry across the world found that more than 55%...
How MedRhythms Captures Clinical Efficacy Data with Castor
MedRhythms used Castor's EDC and eCRF solutions to conduct their pivotal study. Castor's eCRF provides an instant snapshot...
Using Decentralized Trial Technology to Ensure Study Continuity and Data Quality During COVID-19 and Beyond
Download this white paper to learn why patient-centric, remote research technologies such as eConsent, EDC, and ePRO are...

How to manage your study in Castor
During this webinar, we showed how to efficiently manage studies in Castor. This includes: How to add users...

5 steps to meet PMCF requirements
Read the white paper to learn how Castor’s 5-step approach to PMCF can be used to fulfill MDR...

Tips & Tricks for data entry in Castor EDC
This webinar is dedicated to data entry users of Castor EDC. If you are conducting data entry for...

Satisfying PMCF requirements by utilizing IIS data
This white paper describes an approach that would enable medical device manufacturers to leverage anonymous data from IIS...

Protocol Deviations in Clinical Trials: Understanding the FDA’s Guidance
Protocol deviations can seriously impact the integrity of clinical trials, and with regulatory scrutiny growing, it’s crucial to...

All you need to know about randomization in Castor
This webinar teaches everything you need to know about randomization in Castor. This includes: How to randomize records...

In-Vitro Diagnostic Regulation (IVDR): From oversight to overhead
The rising demand for early, accurate disease diagnosis and the growing possibilities in personalised medicine are driving the...

How to effectively monitor studies in Castor EDC
This webinar covers everything you need to know about monitoring in Castor. This includes: Raising and managing queries...
Castor vs. the Coronavirus and COVID-19
Last updated on June 30, 2020 For existing and new Castor customers Castor will continue to deliver its...

6 essential steps to ensure Medical Device Regulation (MDR) compliance before May 2021
July 17th 2020: This document has been updated to reflect regulatory changes in response to COVID-19. As this...

How to create patient surveys in Castor (ePRO/eCOA)
In this webinar, we teach how to send questionnaires / electronic patient-reported outcomes (ePRO) to patients that will...

What does the Medical Device Regulation (MDR) tell us about Post-Market Surveillance?
With the Medical Device Regulation (MDR) coming into full force in 2020, medical device companies should prepare for...